變性血脂是引起血管老化及粥狀硬化的原因
變性血脂是引起蝴蝶斑病人出現血管過早老化及心血管疾病的原因
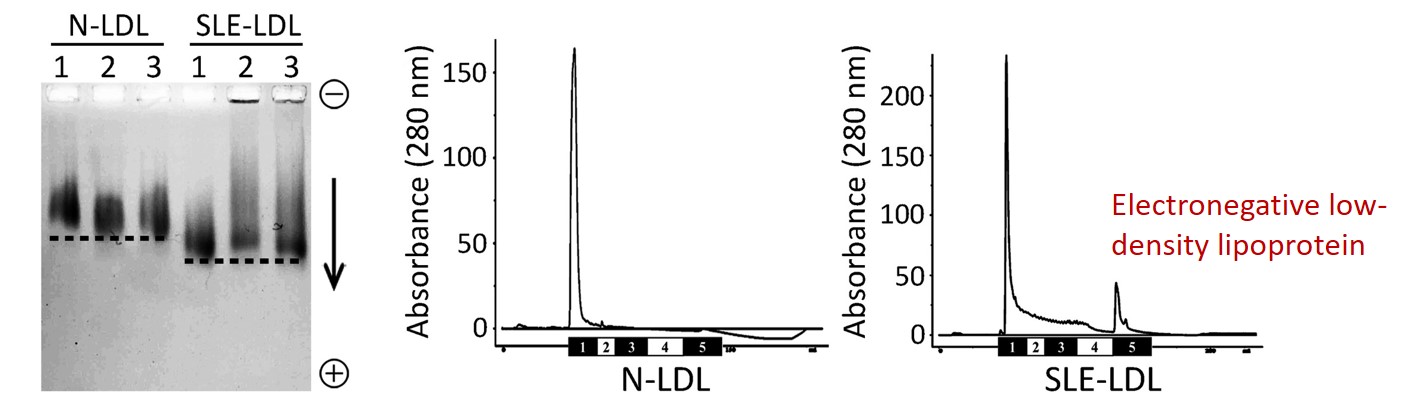
紅斑性狼瘡 (Systemic Lupus Erythematosus; SLE,又稱蝴蝶斑) 好發於30~40歲的年輕族群,血液中壞膽固醇指數 (low-density lipoprotein; LDL) 不高,但卻非常容易發生血管過早老化、動脈粥狀硬化及其他心血管疾病,這是過去臨床醫師沒法解釋的重要議題。高醫附設中和紀念醫院血脂生科研究中心與過敏免疫風濕內科、心臟內科醫師團隊基於陰電性低密度脂蛋白 (electronegative low-density lipoprotein; L5 LDL,又稱變性血脂) 的特色研究,共同探討可能的致病原因。
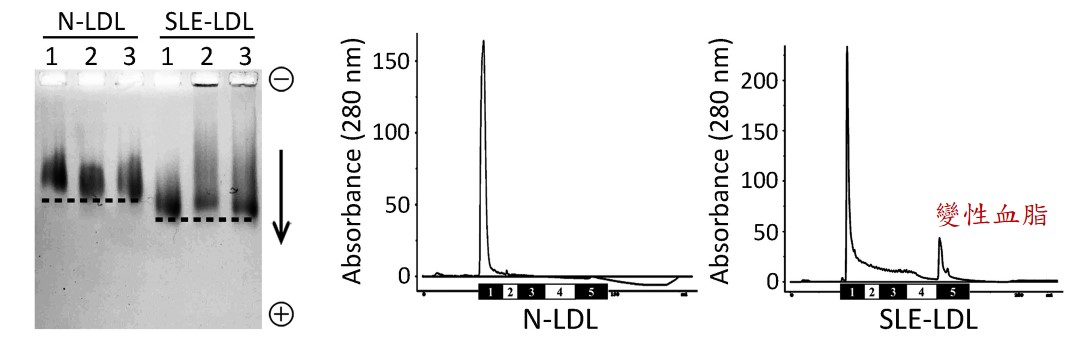
蝴蝶斑病人的變性血脂主要促使發炎反應的成分
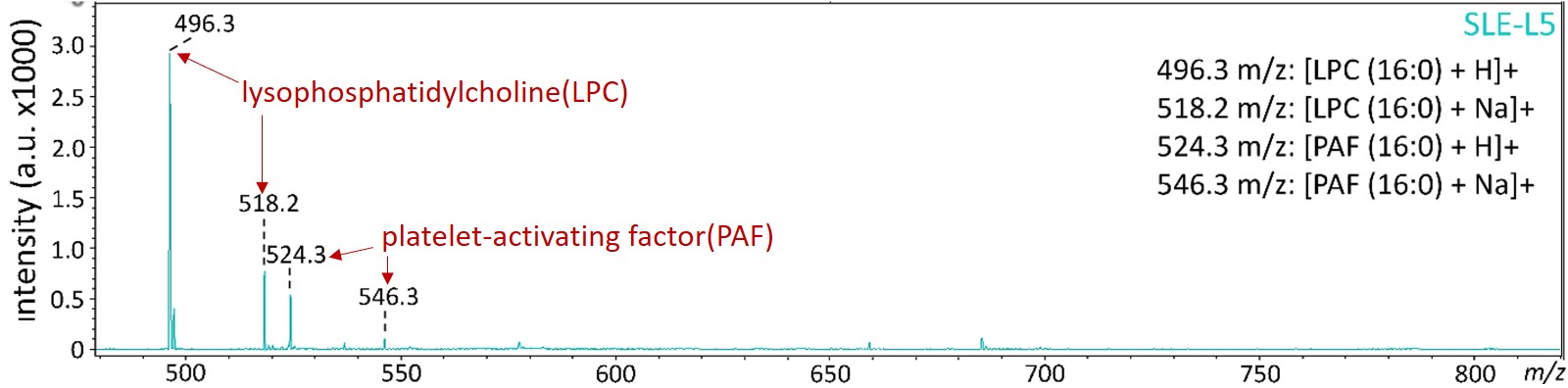
變性血脂的主要特殊成分為高濃度的溶血磷脂 (lysophosphatidylcholine; LPC) 與血小板活化因子 (platelet-activating factor; PAF),這些生物活性脂質容易造成血管內皮細胞老化與細胞凋亡,也會引起免疫細胞與血小板活化,與心腦血管、內分泌代謝、癌症…等疾病都息息相關。其中,溶血磷脂主要是經由磷脂酶 (phospholipase A2)所產生代謝物,而蝴蝶斑病人就是代謝增加或者來不及清除,所以堆積在血液中而引起一連串的發炎病理現象。
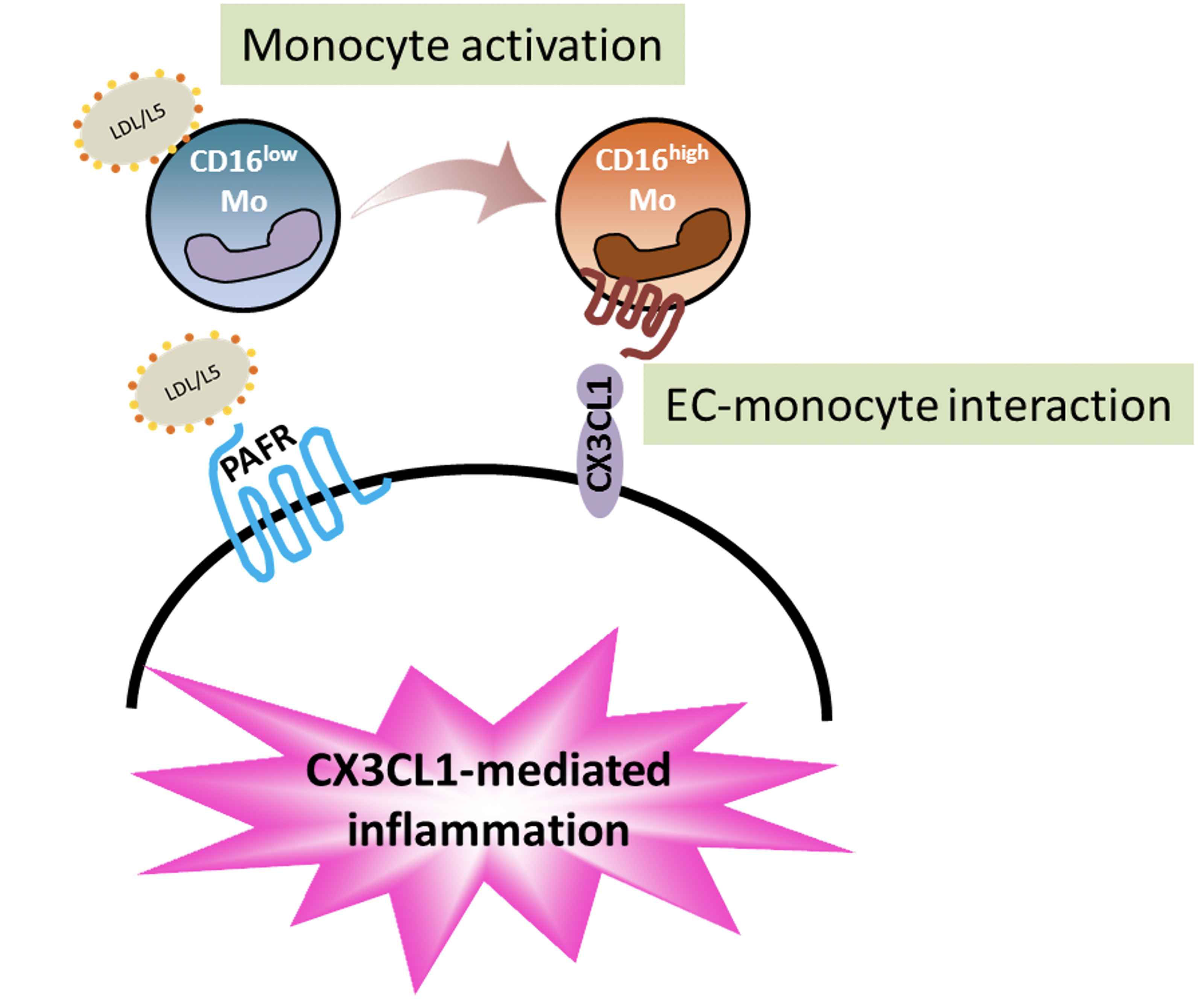
生物活性脂質會增加發炎的白血球與造成血管老化
我們的研究發現,生物活性脂質會讓白血球分化、增生為CD16+單核球細胞(發炎的白血球),同時會破壞血管內皮細胞,透過兩種細胞的交互作用 (CX3CL1-CX3CR1),發炎的白血球會浸潤在血管內皮層,讓血管功能失調、血管老化。這個病理現象可以解釋為什麼蝴蝶斑病人往往有心血管疾病的病發症,也提供可以改變未來治療的策略。
結論
蝴蝶斑病人的變性血脂濃度增加,並具有高濃度的生物活性的脂質溶血磷脂與血小板活化因子,這會引起白血球分化成傾向發炎反應的細胞,並導致血管內皮細胞活化,透過分子交互作用使血管功能失調與血管老化。這個病理現象的發生不會侷限於蝴蝶斑病人,倘若任何民眾有心血管疾病的疑慮,擔心血管老化及血管粥樣硬化,應到醫學中心安排進一步檢查血管功能及變性血脂濃度,並諮詢醫師研擬個人化的治療策略。
圖形摘要
應用與亮點
1.蝴蝶斑病人的變性血脂濃度增加,具有高濃度的生物活性的脂質–溶血磷脂與血小板活化因子。
2.變性血脂引起白血球分化成傾向發炎反應的細胞,也會讓血管內皮細胞受傷,藉由兩種細胞的交互作用CX3CL1-CX3CR1使血管功能失調與老化。
3.若有心血管疾病的疑慮,擔心血管老化及血管粥樣硬化,應到醫學中心安排進一步檢查血管功能及變性血脂濃度,並諮詢醫師研擬個人化的治療策略。
【研究團隊】
團隊成員:柯良胤、顏正賢
代表單位:健康科學院/醫學檢驗生物技術學系
團隊簡介:醫技系柯良胤副教授與過敏免疫風濕內科顏正賢教授、血脂生科研究中心詹華蓁博士…等人共同發表的研究論文「Role of low-density lipoprotein in early vascular aging associated with systemic lupus erythematosus」,該論文受到Nature Reviews 期刊編輯Joanna Clarke的關注與採訪,並隨即發表於Nature Reviews Rheumatology研究亮點,文章題目為LDL subfraction linked to vascular ageing and heart disease in SLE。
研究聯繫Email:kly@gap.kmu.edu.tw
【論文資訊】
論文出處:Arthritis & Rheumatology 2020 June.72(6):972-984.
全文下載:https://onlinelibrary.wiley.com/doi/epdf/10.1002/art.41213
Role of low-density lipoprotein in early vascular aging associated with systemic lupus erythematosus
Role of low-density lipoprotein in early vascular aging associated with systemic lupus erythematosus
L5 LDL linked to vascular aging in patients with Systemic Lupus Erythematosus (SLE)
SLE patients often have atherosclerotic complications at a young age but normal low-density lipoprotein (LDL) levels. Thus, this study was aimed to explain the paradoxical findings of early vascular aging (EVA) and normolipidemia in patients with SLE. In KMU, we focused on a different approach: the electronegative LDL. Human plasma LDL can be separated into five subfractions. L5 LDL, in particular, has been shown to be atherogenic, independent of LDL cholesterol. From here, we investigated the mechanisms of vascular aging in SLE patients.
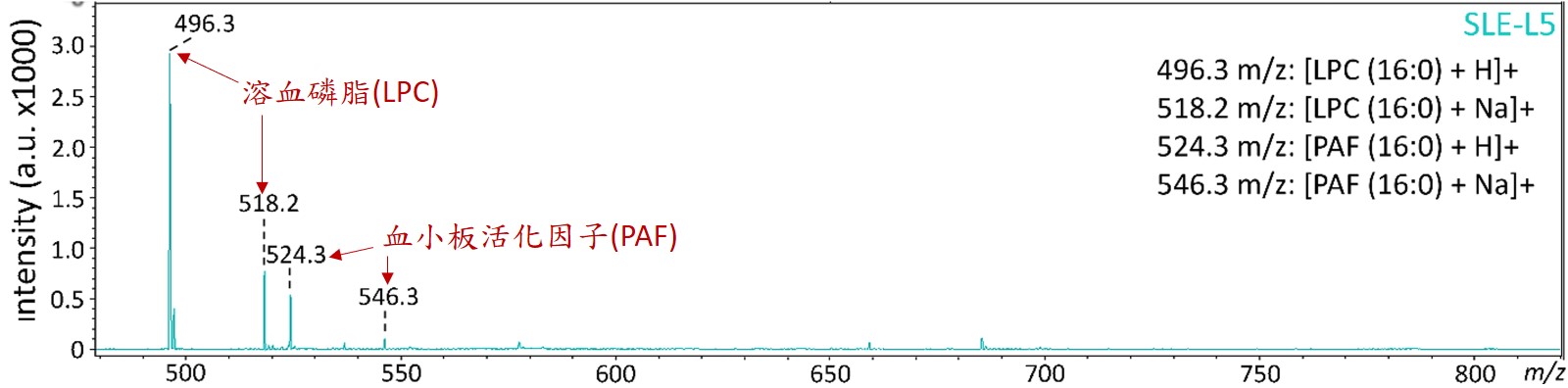
The mass spectrometric analysis revealed that lysophosphatidylcholine (LPC) and platelet-activating factor (PAF) were increased in SLE-LDL and L5.
LPC and PAF are bioactive lipids, showing the capability to induce inflammatory changes in vascular cells both in vivo and in vitro. In this study, repeated injections of SLE-LDL, LPC or PAF to apoE-/- mice led to increases in IMT, collagen deposition, fatty-streak areas in the aortic wall, and cell senescence in the aortic endothelium.
SLE-L5 induces CD16+ expression in monocytes and monocyte adhesion to ECs through CX3CL1-CX3CR1 interactions.
Flow cytometric analysis showed that SLE-L5 lipids, but not SLE-L1 lipids, induced an increase in the percentage of CD16+ monocytes, which are CX3CR1 expressing cells. On the other hand, SLE-LDL induces CX3CL1 expression in activated endothelial cells. Through CX3CL1-CX3CR1 interactions, SLE-L5 induces early atherosclerotic changes in young apoE-/- mice. SLE-L5 induced significant atherosclerotic lesion formation in the aortic arch of the mice as well as collagen deposition and elastic fragmentation in aortas.
Conclusion
L5 LDL (the most electronegative subfraction) is increased in SLE patients. By mass spectrometry, results showed that L5 LDL is an LPC- and PAF-rich lipoprotein. Through CX3CL1-CX3CR1 interactions between ECs and CD16+ monocytes, L5 LDL promotes atherosclerosis. Our findings suggest that the L5 level, not the total LDL concentration, should be a therapeutic target for preventing EVA and atherosclerosis in patients with SLE.
Graphical Abstract

Application and Highlights:
1. Through CX3CL1-CX3CR1 interactions between ECs and CD16+ monocytes, L5 LDL promotes atherosclerosis.
2. An increase in plasma L5 levels, not total LDL concentration, may promote early vascular aging in SLE patients, leading to premature atherosclerosis.
3. The L5 level should be a therapeutic target for preventing early vascular aging and atherosclerosis in patients with SLE.
Research Team Members:
Liang-Yin Ke, Jeng-Hsien Yen
Representative Department:
Department of Medical Laboratory Science and Biotechnology, College of Health Sciences
Introduction of Research Team:
Liang-Yin Ke (from the Department of Medical Laboratory Science and Biotechnology), Jeng-Hsien Yen (from Graduate Institute of Medicine), the first author Hua-Chen Chan (Center for Lipid Biosciences) and colleagues published the above-named paper in the Arthritis & Rheumatology this year. Immediately, the paper got great attention. The editor of Nature Reviews, Joanna Clarke, interviewed with the corresponding authors, and later she wrote a “research highlight” editorial and published in Nature Reviews Rheumatology, entitled as “LDL subfraction linked to vascular aging and heart disease in SLE.”
Contact Email:kly@gap.kmu.edu.tw
Publication: Arthritis Rheumatol. 2020 Jun;72(6):972-984.
Full-Text
Article: https://onlinelibrary.wiley.com/doi/epdf/10.1002/art.41213





