口腔鱗狀細胞癌治療的新契機
口腔鱗狀細胞癌治療的新契機
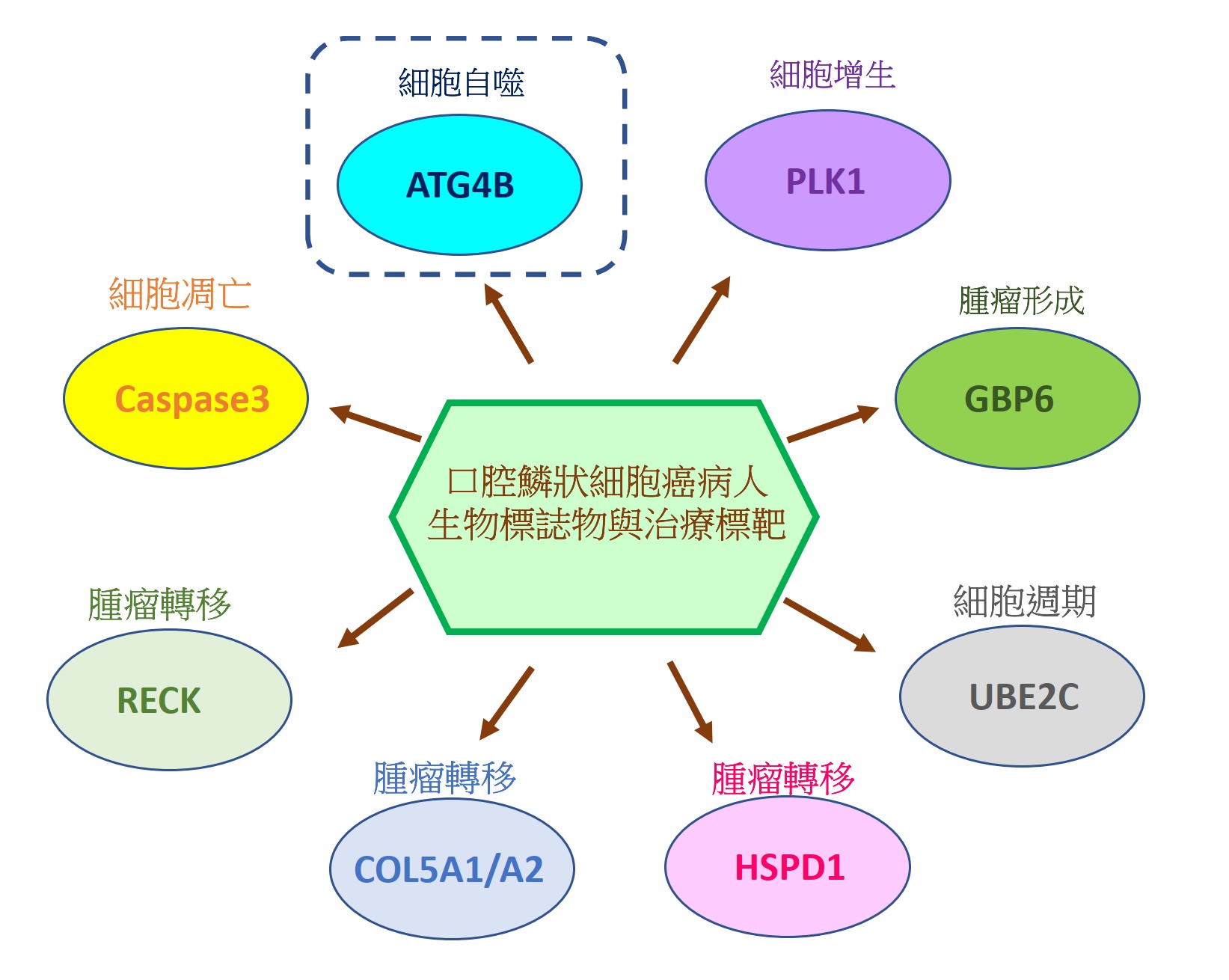
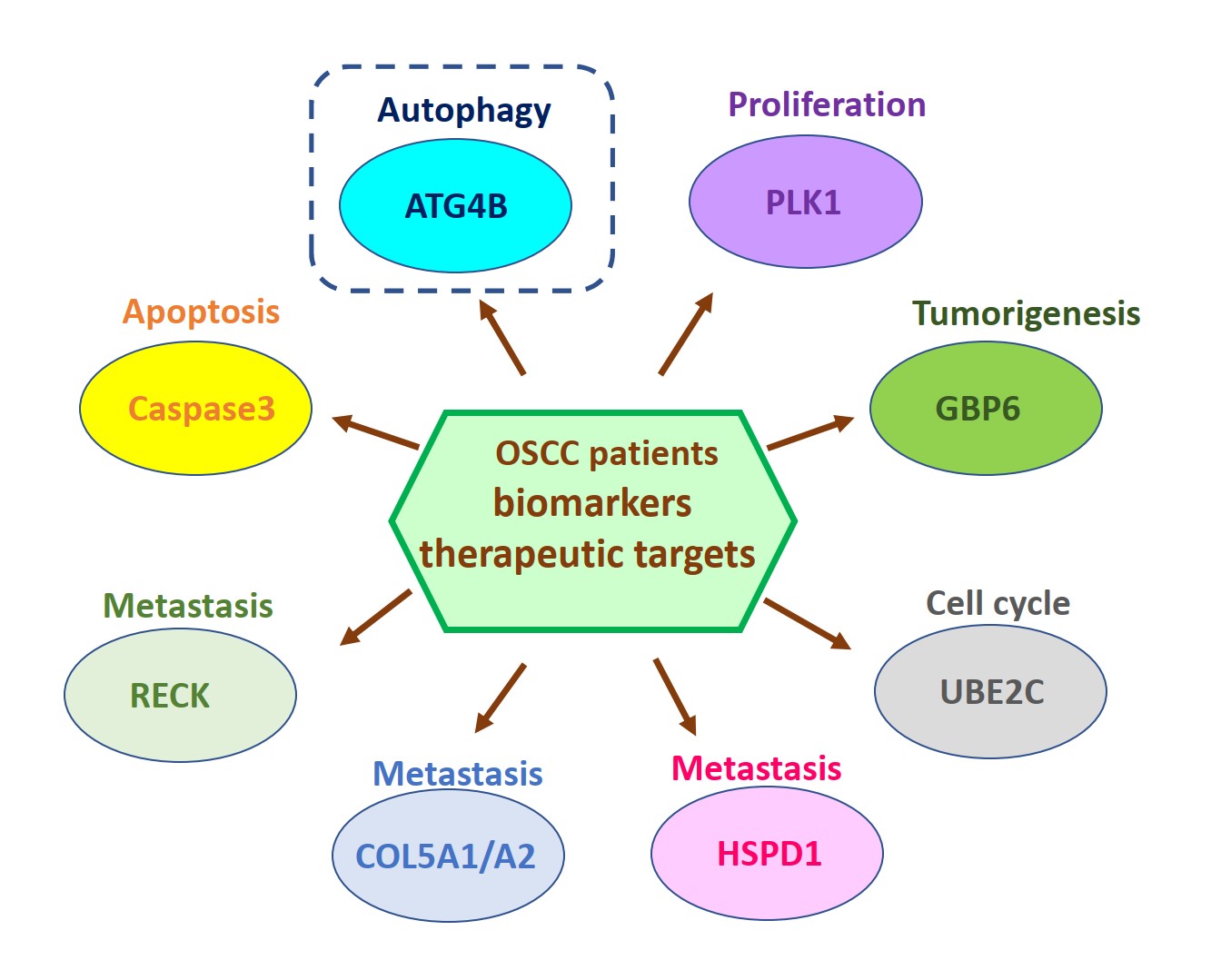
由於缺乏潛在的生物標誌物和治療靶標,導致口腔鱗狀細胞癌(OSCC)仍然是全世界癌症死亡的主要原因之一。因此,我們的研究重點放在找出具潛力的OSCC生物標誌物和治療靶標。我們已經從癌症基因組圖譜數據庫中分析口腔癌患者組織中的基因表現情形或進行siRNA庫篩選,藉由定量聚合酶鏈反應和免疫組織化學來比較OSCC患者正常及腫瘤組織中的基因表現來確認潛在的致癌基因和抑癌基因。我們還透過OSCC癌細胞和異種移植小鼠模型驗證了這些潛在生物標記物的作用和分子機制。到目前為止,我們已經發表幾篇具潛力的OSCC生物標誌物和治療靶標相關論文,如圖1所示。在這裡,我們介紹其中一個具潛力的生物標誌物和治療靶標-自噬相關蛋白酶4B(ATG4B),如圖2所示。
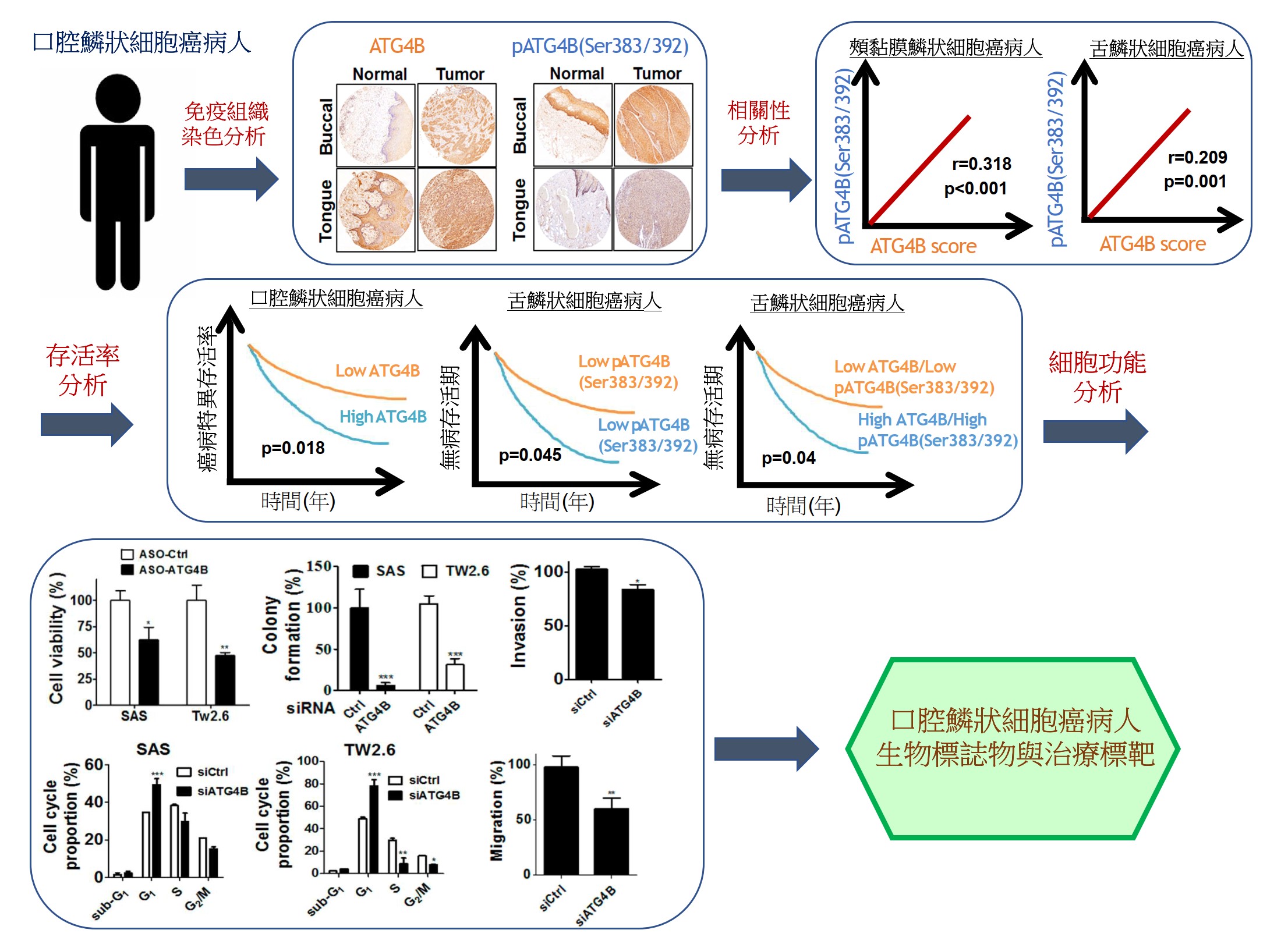
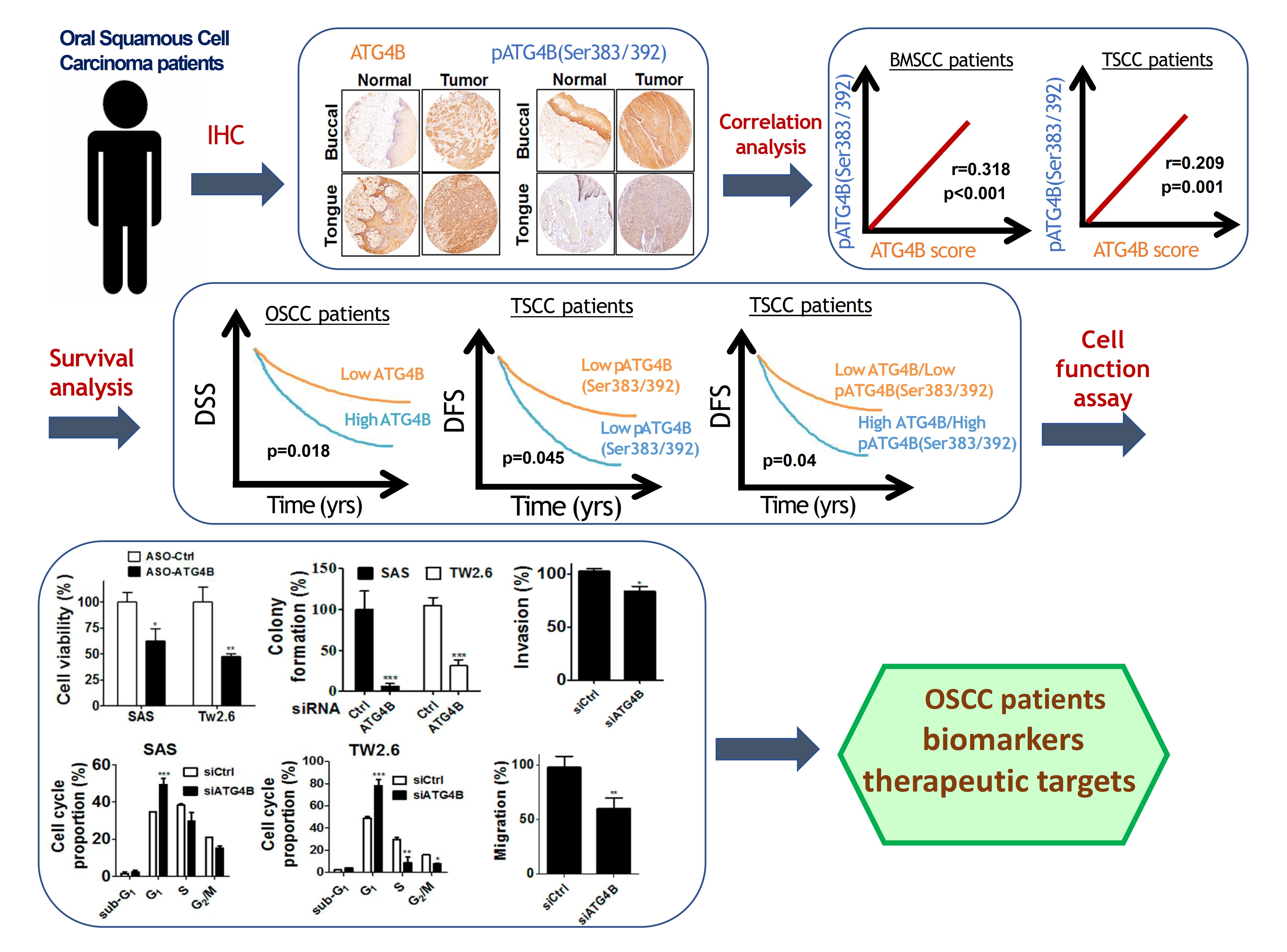
自噬相關蛋白酶4B(ATG4B)是自噬作用中不可少的蛋白酶,Ser383/392處磷酸化的ATG4B可增加其蛋白水解活性。 ATG4B的表現和活化對於癌細胞的增殖和侵襲相當重要。然而,ATG4B和Ser383/392處磷酸化的ATG4B在OSCC患者臨床之關聯性仍然未知,尤其在頰黏膜SCC(BMSCC)和舌頭SCC(TSCC)患者中。使用498例OSCC患者檢體做成組織微陣列,包括179例BMSCC和249例TSCC患者,我們發現BMSCC和TSCC患者腫瘤組織中的ATG4B和Ser383/392處磷酸化的ATG4B之蛋白表現量比鄰近組織正常中的要高。在OSCC患者中,特別是在晚期腫瘤患者中,高蛋白表現量的ATG4B與較差的疾病特異性生存率(DSS)有顯著相關。另外,Ser383/392處磷酸化的ATG4B1蛋白表現量與TSCC患者的不良無病生存率(DFS)也相關。此外,在BMSCC和TSCC患者中,ATG4B蛋白表達量與Ser383/392處磷酸化ATG4B蛋白表現量呈現正相關。然而,僅在TSCC患者中,同時高蛋白表現量的ATG4B和Ser383/392處磷酸化的ATG4B與患者較差的DFS相關,而在BMSCC和TSCC患者中,它們卻與患者DSS無顯著相關。此外,用反義寡核苷酸(ASO)或干擾RNA(siRNA)沉默ATG4B可以減少TW2.6和SAS口腔癌細胞的細胞增殖。另外,剔除口腔癌細胞的ATG4B可以減少細胞遷移和侵襲。綜上所述,這些發現顯示,ATG4B可能作為未來OSCC患者的潛在生物標誌物和治療靶標。
本校主要研究者之簡介:
劉佩芬助理教授(生物醫學暨環境生物學系)
研究聯繫Email:
pfliu@kmu.edu.tw
期刊出處:
Cancers 2019, 11(12), 1854
研究全文下載:
https://www.mdpi.com/2072-6694/11/12/1854
New opportunities for the oral squamous cell carcinoma treatment
New opportunities for the oral squamous cell carcinoma treatment
Oral squamous cell carcinoma (OSCC) remains one of the major leading causes of cancer death worldwide due to the lack of potential biomarkers and therapeutic targets. Thus, our research is focusing on identifying potential biomarkers and therapeutic targets for OSCC. We have analyzed the gene expression in the tissues of oral cancer patients from the Cancer Genome Atlas database or performed siRNA library screening to identify potential oncogenes and tumor suppressive genes by comparing gene expression between normal and tumor tissue of OSCC patients with quantitative polymerase chain reaction and immunohistochemistry. We also verified roles and molecular mechanisms of these potential biomarkers by using OSCC cancer cells and xenografted mice models. So far, we have published several related papers with potential OSCC biomarkers and therapeutic targets, as shown in Figure 1. Here, we introduced one of the potential biomarkers and therapeutic targets-autophagy-related protease 4B (ATG4B), as shown in Figure 2.
Autophagy related protease4B (ATG4B) is an essential protease for the autophagy machinery, and ATG4B phosphorylation at Ser383/392 increases its proteolytic activity. ATG4B expression and activation are crucial for cancer cell proliferation and invasion. However, the clinical relevance of ATG4B and phospho-Ser383/392-ATG4B for OSCC remains unknown, particularly in buccal mucosal SCC(BMSCC) and tongue SCC (TSCC). With a tissue microarray comprising specimens from 498 OSCC patients, including 179 BMSCC and 249 TSCC patients, we found that the protein levels of ATG4B and phospho-Ser383/392-ATG4B were elevated in the tumor tissues of BMSCC and TSCC compared with those in adjacent normal tissues. High protein levels of ATG4B were significantly associated with worse disease-specific survival (DSS) in OSCC patients, particularly in patients with tumors at advanced stages. In contrast, phospho-Ser383/392-ATG4B expression was correlated with poor disease-free survival (DFS) in TSCC patients. Moreover, ATG4B protein expression was positively correlated with phospho-Ser383/392-ATG4B expression in both BMSCC and TSCC. However, high coexpression levels of ATG4B and phospho-Ser383/392-ATG4B were associated with poor DFS only in TSCC patients, whereas they had no significant association with DSS in BMSCC and TSCC patients. In addition, silencing ATG4B with an antisense oligonucleotide (ASO) or small interfering RNA (siRNA) diminished cell proliferation of TW2.6 and SAS oral cancer cells. Further, knockdown of ATG4B reduced cell migration and invasion of oral cancer cells. Taken together, these findings suggest that ATG4B might be a potential biomarker and therapeutic target for OSCC patients in the future.
Main researcher Intro.
Assistant Professor Dr. Pei-Feng Liu
(Department of Biomedical Science and Environment Biology)
Author Email:
pfliu@kmu.edu.tw
Paper cited from:
Cancers 2019, 11(12), 1854
Research Paper available online on website:
https://www.mdpi.com/2072-6694/11/12/1854




