慢性C型肝炎患者使用Sofosbuvir為基底小分子抗病毒藥物治療對其腎功能之影響-TACR註冊系統全國性研究
慢性C型肝炎患者使用Sofosbuvir為基底小分子抗病毒藥物治療對其腎功能之影響-TACR註冊系統全國性研究
C型肝炎病毒 (HCV) 根據全球統計是導致肝細胞癌與肝臟相關疾病死亡的主要原因之一。在台灣C型肝炎感染也是引起肝硬化及肝癌的主要病因之一,其比例僅次於B型肝炎。由於HCV具自體免疫和淋巴增生的特性,因此慢性C型肝炎(CHC) 患者經常伴隨著其他肝外表徵,如會有較高風險的慢性腎臟疾病(CKD)或導致末期腎病。自從新型C型肝炎口服抗病毒藥物(DAA)上市後,可有效清除95%以上之HCV,副作用少、療程大幅縮短且方便服用,可說是C型肝炎患者一大福音。Sofosbuvir (SOF)是常見C肝抗病毒用藥之一,其主要是透過腎臟排除,在腎功能不好的患者其代謝產物 GS331007會大量累積在體內。雖然在輕、中度腎功能不全病人可安全使用,但在重度腎功能不全的病人於本文章發表之前其安全性還沒有完全確立。因此在C型肝炎感染伴隨CKD患者,給予SOF治療其對腎功能的影響仍待進一步釐清。
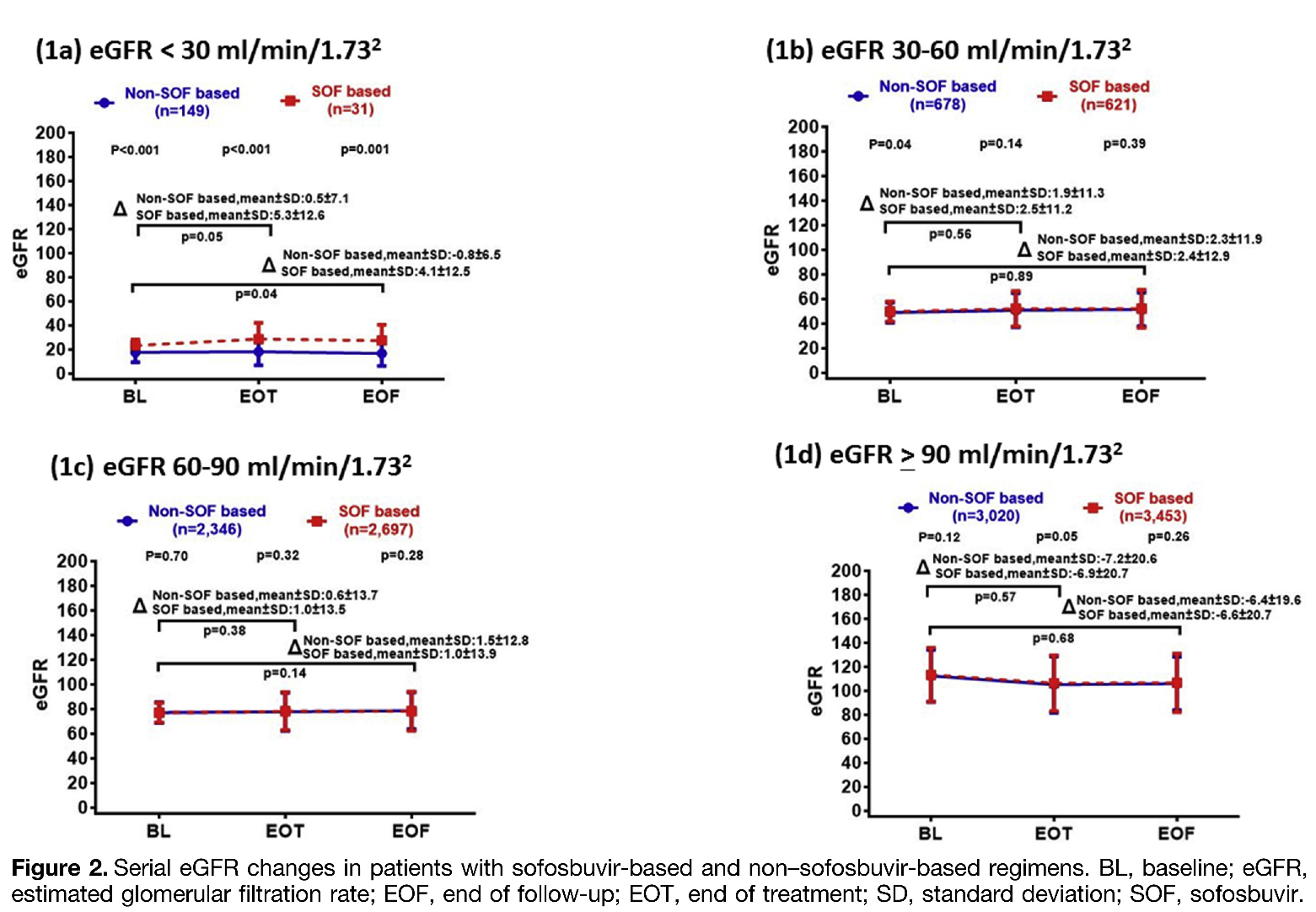
高雄醫學大學醫學院肝炎研究中心及液態生物檢體暨世代研究中心團隊,透過TACR全國性37家醫學中心、區域醫院及地區醫院C肝註冊平台收集全國12,995個C型肝炎患者臨床個案,分別為給予含SOF治療者(n=6,802) 及非給予SOF治療者(n=6,193),追蹤調查其治療前、治療結束及結束後三個月的腎絲球過濾率(eGFR)變化,全面性分析評估SOF抗病毒藥物治療CHC尤其是伴隨CKD患者,對其腎功能是否有所影響。研究結果顯示,CHC伴隨CKD患者(eGRF < 60 mL/min/1.73 m2)接受SOF治療過程,無論任何階段其腎功能都不會受到影響,且與非SOF治療者一樣在病毒清除後腎功能eGFR會顯著改善。有趣的是,在重度腎功能不全eGRF < 30 mL/min/1.73 m2的CHC病人接受SOF治療後,其腎功能改善的斜率更勝非接受SOF治療的患者。本全國性研究根據大規模調查,證實以SOF為基底抗病毒藥物,不僅可有效治療C型肝炎,且對CKD患者不會造成腎功能的負擔甚至改善,提供了CHC患者治療的實證醫學證據。
圖形摘要
應用與亮點:
1. 成功的將HCV清除可顯著降低肝臟相關併發症風險,如改善腎功能。
2. Sofosbuvir (SOF) 為基底的治療可以安全地應用於末期腎臟疾病的患者。
【研究團隊】
團隊成員:黃釧峰教授、余明隆教授、莊萬龍教授、戴嘉言教授、黃志富教授、葉明倫副教授。
代表單位:液態生物檢體暨世代研究中心及醫學院肝炎研究中心
團隊簡介:黃釧峰教授與余明隆教授研究團隊,皆任職於是高雄醫學大學附設中和紀念醫院肝膽胰內科的主治醫師,同時也是醫學院肝炎研究中心及液態生物檢體暨世代研究中心的成員,致力於肝臟疾病之病因、致病機轉、治療及預防的研究及服務。
研究聯繫Email:fengcheerup@gmail.com
【論文資訊】
論文出處: Clin Gastroenterol Hepatol. 2022 May;20(5):1151-1162.e6. doi:10.1016/j.cgh.2021.07.037. Epub 2021 Jul 30.
全文下載: https://doi.org/10.1016/j.cgh.2021.07.037
Impact of Sofosbuvir-Based Direct-Acting Antivirals on Renal Function in Chronic Hepatitis C Patients With Impaired Renal Function: A Large Cohort Study From the Nationwide HCV Registry Program (TACR)
Impact of Sofosbuvir-Based Direct-Acting Antivirals on Renal Function in Chronic Hepatitis C Patients With Impaired Renal Function: A Large Cohort Study From the Nationwide HCV Registry Program (TACR)
Hepatitis C virus (HCV) is one of the leading etiologies of hepatocellular carcinoma and liver-related mortality worldwide. Because of their highly effective and well-tolerated properties, direct-acting antivirals (DAAs) are the current standard of care in treating chronic hepatitis C (CHC). DAAs are metabolized exclusively by the liver. Sofosbuvir (SOF) is extensively metabolized to the active nucleoside analog triphosphate GS-461203 in hepatocytes. Finally, the inactive metabolite GS-331007 is mainly eliminated through the renal route. The accumulation and delayed excretion of GS-331007 have been documented in patients with poor renal function, and the use of SOF-based regimens has raised the issue of potential nephrotoxicity in patients with severe renal disease. Herein, we aimed to address this issue by conducting a nationwide study including CHC patients who were treated with SOF-based or non–SOF-based regimens across all CKD stages.
The 12,995 CHC patients treated with sofosbuvir-based (n= 6,802) or non–sofosbuvir-based (n =6,193) regimens were retrieved from the Taiwan nationwide real-world HCV Registry Program (TACR). Serial estimated glomerular filtration rate (eGFR) levels were measured at baseline, end of treatment (EOT), and end of follow-up (EOF) (3 months after EOT). A universal increase in eGFR, especially in patients with eGFR <60 mL/min/1.73 m2, confirms the benefit of DAAs in promoting renal function recovery. Intriguingly, among patients whose baseline eGFR was <30 mL/min/1.73 m2, the magnitude of increased eGFR at EOT or EOF was more pronounced in patients receiving SOF-based regimens than in those receiving non–SOF-based regimens. The slope coefficient difference between patients with SOF-based and non-SOF-based regimens existed only in patients with baseline eGFR <30 mL/min/1.73 m2. In conclusion, sofosbuvir and non-sofosbuvir-based regimens restored renal function in CHC patients with CKD, especially those with significant renal function impairment.
Graphical Abstract

Application and Highlights:
1.HCV eradication by direct-acting antivirals restores renal function, particularly in patients with chronic kidney disease.
2.Sofosbuvir-based regimens could be safely applied in patients with severe renal disease in the real-world setting.
Research Team Members:
Chung-Feng Huang, Ming-Lung Yu, Wan-Long Chuang, Chia-Yen Dai, Jee-Fu Huang, Ming-Lun Yeh
Representative Department:
Center for Liquid Biopsy and Cohort Research, Kaohsiung Medical University, and Hepatitis Research Center, College of Medicine, Kaohsiung Medical University.
Introduction of Research Team:
Professor Chung-Feng Huang and Professor Ming-Lung Yu are members of the Hepatitis Center and Hepatobiliary Division, Department of Internal Medicine, Kaohsiung Medical University Hospital and Hepatitis Research Center, College of Medicine, Kaohsiung Medical University, and Center for Liquid Biopsy and Cohort Research, Kaohsiung Medical University. They dedicate to the research and service of liver diseases, including etiologies, epidemiology, pathogenesis, therapeutic modalities, and prevention of hepatitis B, hepatitis C, steatohepatitis, and liver cancers.
Contact Email: fengcheerup@gmail.com
Publication: Clin Gastroenterol Hepatol. 2022 May;20(5):1151-1162.e6. doi:10.1016/j.cgh.2021.07.037. Epub 2021 Jul 30.
Full-Text Article: https://doi.org/10.1016/j.cgh.2021.07.037

