亞洲慢性C型肝炎患者接受治療小分子抗病毒藥物REAL-C大規模真實世界研究
亞洲慢性C型肝炎患者接受治療小分子抗病毒藥物REAL-C大規模真實世界研究
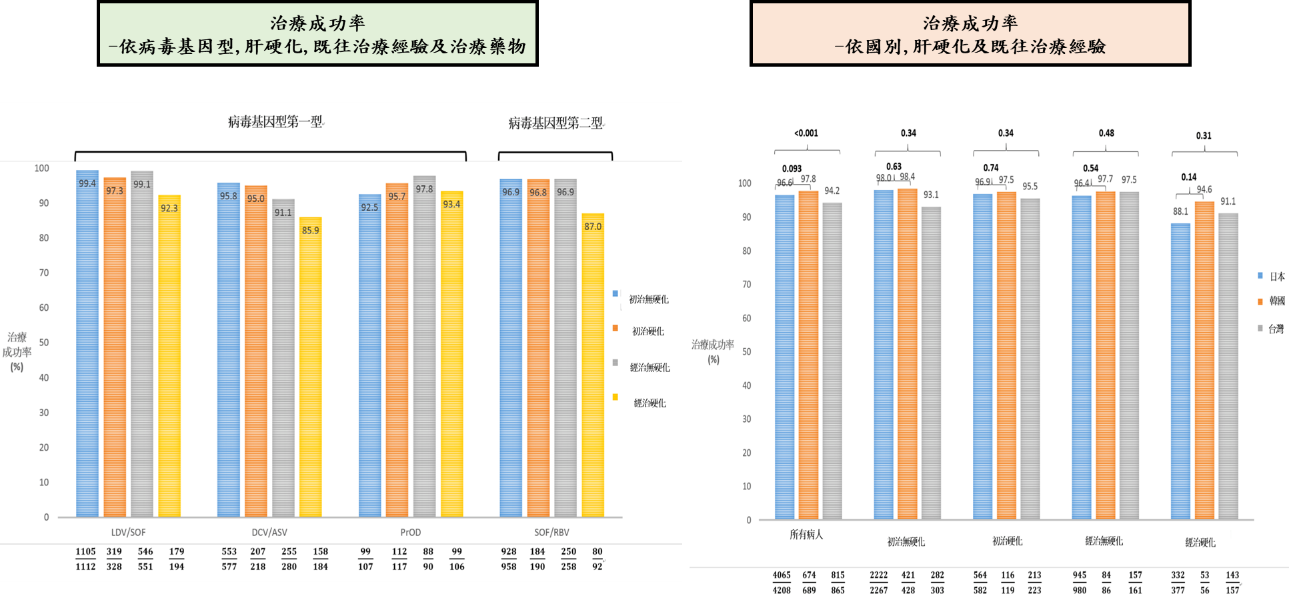
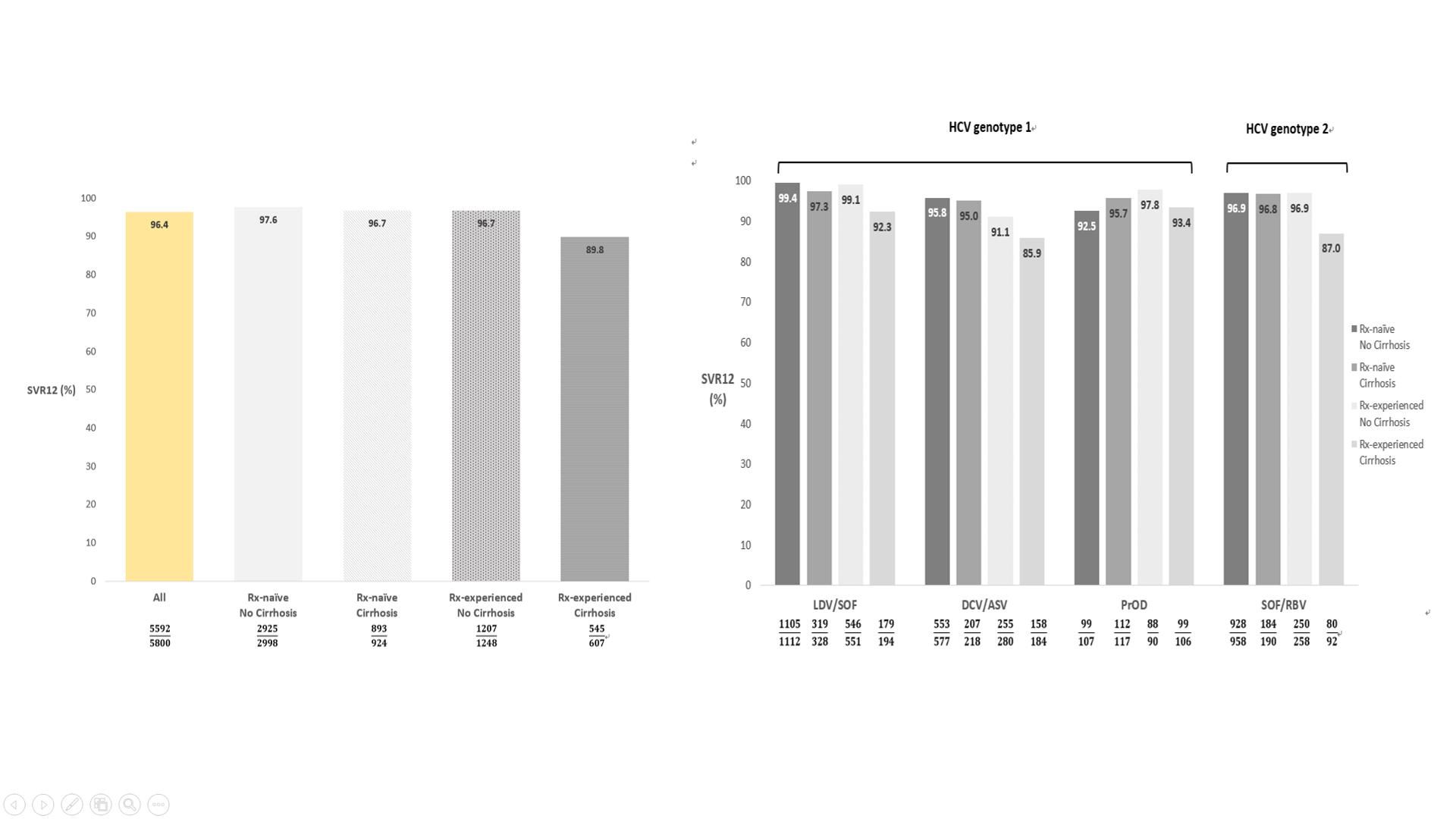
全球有1/3的C型肝炎患者位於亞洲,慢性C型肝炎小分子抗病毒藥物(DAA)既往亞洲真實世界報告多為日本單中心或單一醫療體系發表。亞洲C型肝炎分布人口學及地域分佈複雜,在亞洲多國綜觀的大規模治療報告仍付之闕如。此研究針對亞洲多國慢性C型肝炎患者接受小分子抗病毒藥物的真實世界療效及安全性作進一步探討。REAL-C (Real-world Evidence from the Asia Liver consortium for HCV)為亞洲多國包含台灣、日本、韓國及香港超過20個多中心大規模C型肝炎研究聯盟註冊系統(Registry),此研究的主要目的為探討亞洲C型肝炎族群小分子抗病毒藥物療效(持續性病毒反應;定義為抗病藥物停藥後12週體內偵測不到病毒;SVR12)及安全性研究,以及探討新藥治療失敗原因。本研究收錄來自台灣、日本、韓國及香港一共6,287位慢性C型肝炎患者接受小分子抗病毒藥物。相較其他國家患者,日本患者年紀較老、身體質量比少,且有較高比例非肝癌惡性腫瘤。整體亞洲族群C肝治療療效為96.4%,且無種族之間差別。不同藥物及不同肝病嚴重程度整體治療成果在91.1%至99.4%之間,唯病毒基因型第一型肝硬化干擾素治療失敗患者使用daclatasvir/asunaprevir(87%)及第二型肝硬化干擾素治療失敗患者使用sofosbuvir/ribavirin(85.9%)有較差治療療效。整體治療中斷率為1.9%且無種族之間差別。多變項結果顯示與治療失敗有關因素為高病毒量、肝硬化及之前DAA治療失敗經驗。種族地域差異非影響治療療效之獨立預測因子。此多國多中心研究顯示小分子抗病毒藥物可有效及安全治療亞洲慢性C型肝炎患者,此實證醫學證據可對於疾病負擔沉重的亞洲國家提供C肝治療真實世界證據及各國C肝公衛政策制定之參考。
本篇為高雄醫學大學2019年月傑出論文9月份得獎研究,代表作者為肝炎研究中心黃釧峰教授。
本校主要研究者之簡介:
黃釧峰 教授
高醫 肝膽內科
高醫大內科學科
研究聯繫Email:
fengcheerup@gmail.com
期刊出處:
Hepatol Int. 2019 Sep;13(5):587-598.
研究全文下載:
https://link.springer.com/article/10.1007/s12072-019-09974-z
Direct-acting antivirals in East Asian hepatitis C patients: Real-world experience from the REAL-C Consortium Background & Aims
Direct-acting antivirals in East Asian hepatitis C patients: Real-world experience from the REAL-C Consortium Background & Aims
One-third of the global hepatitis C virus (HCV) burden is found in Asia. Real-world data from diverse East Asian cohorts remain limited. This study addressed the real-world status of direct-acting antiviral (DAA) therapy among patients from East Asia.
Methods
Chronic hepatitis C (CHC) patients from clinical sites in Japan, Taiwan, South Korea and Hong Kong were recruited in the REAL-C registry, an observational chart review registry. The primary outcome was sustained virologic response (SVR12, HCV RNA PCR .
Results
A total of 6,287 CHC patients were enrolled. Compared to other East Asian patients, patients from Japan were older (66.3 vs. 61.5 years, P<0.0001), had lower body mass indices (22.9 kg/m2 vs. 24.6 kg/m2, P<0.001), and were more likely to have non-liver malignancy history (12.2% vs. 5.0%, P<0.001).The overall SVR12 rate was 96.4%, similar to patients both inside and outside Japan (96.6% vs. 96%, P=0.21). The SVR12 rate ranged from 91.1-99.4% except treatment-experienced cirrhotic HCV genotype-1 patients who received daclatasvir/asunaprevir (85.9%) and the treatment-experienced cirrhotic HCV genotype-2 patients treated with sofosbuvir/ribavirin (87%). The overall rate of drug discontinuation was 1.9%, also similar across regions. Logistic regression analysis revealed that significant independent factors predictive of treatment failure were higher HCV RNA levels (odds ratio [OR], 95%CI: 0.73, 0.59-0.90; P=0.004), the presence of liver cirrhosis (0.68, 0.49-0.84, P=0.046), prior treatment failure with IFN (0.57, 0.42-0.79, P=0.001), and prior treatment failure with DAA other than BOC and TVR (0.04, 0.02-0.08, P<0.001), but not geographic region (non-Japan versus Japan, 1.09, 0.74-1.60, P=0.68).
Conclusions
In this large multinational CHC cohort from the East Asia, oral DAAs were highly effective and well-tolerated across the region. Policies should encourage treatment for all CHC patients with DAAs in Asia with its heavy burden of HCV.
Graphical Abstract
This article-“Direct-acting antivirals in East Asian hepatitis C patients: real-world experience from the REAL-C Consortium” , written by Rept. Author researcher Chung-Feng Huang, from Hepatitis Research Center, is award for Kaohsiung Medical University 2019 Monthly Excellent Paper Award in Sept.
Main researcher Intro.
Researcher :
Chung-Feng Huang MD.PhD.
Hepatobiliary Division, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
Author Email
fengcheerup@gmail.com
Paper cited from:
Hepatol Int. 2019 Sep;13(5):587-598.
Research Paper available online on website https://link.springer.com/article/10.1007/s12072-019-09974-z


