高醫團隊發現口腔癌新致病機轉及潛在治療方式
高醫團隊發現口腔癌新致病機轉及潛在治療方式
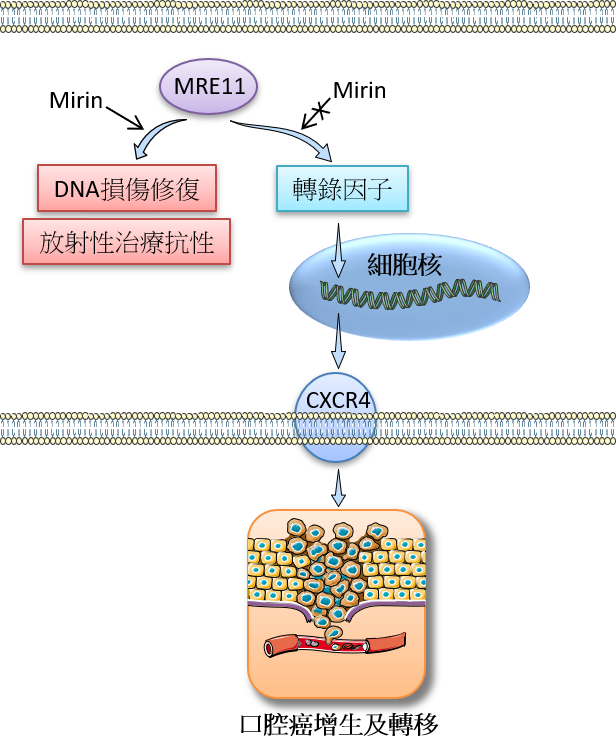
口腔癌在台灣是癌症致死的主要原因之一,尤其好發在台灣男性族群。然而,它的生物機轉及專一性治療策略仍然有待進一步的研究。MRE11是一種與去氧核醣核酸(DNA)的損傷修復有關的重要蛋白,其核酸水解酶活性已知與多種癌症的發展有關連性;然而,MRE11的非核酸水解酶功能卻鮮為人知。在這篇研究報告中,我們新發現MRE11的非核酸水解酶活性與口腔癌的發展有密切相關,並且證實此相關性是透過調控化學趨化受體CXCR4的表現量及其訊息傳遞來完成。
我們首先發現口腔癌病人檢體中的MRE11表現量高低與其腫瘤大小、癌症進展的分期和癌細胞轉移淋巴結之間有正相關,而且MRE11表現量越高的口腔癌病人其預後越差,並易於對放射性治療產生抗性導致治療結果不佳。我們接著用細胞及動物實驗探討這些臨床症狀的生物機轉,發現較多的MRE11會促進CXCR4的表現,並透過其訊息傳遞路徑的活化造成口腔癌細胞的增生及轉移。我們進一步發現這些現象並不是來自MRE11的核酸水解酶活性,而是來自其非核酸水解酶活性,因為當我們加入一種稱為mirin(專門用來抑制MRE11核酸水解酶活性)的抑制劑時,並不會影響經由MRE11所引起的口腔癌細胞增生及轉移的現象。此外,當我們加入CXCR4抗體於細胞或動物實驗時,皆發現此經由MRE11所引起的口腔癌細胞增生及轉移的現象被抑制了。
綜合上述,調控MRE11的表現量將可能成為治療口腔癌的一個新的標的,我們也預期本研究成果能成為臨床診斷口腔癌之重要參考。此外,未來也許可以根據口腔癌病人不同的MRE11表現量發展出個人化的醫療策略。
應用與亮點:
- 口腔癌組織中高表現量的MRE11與口腔癌惡化指標以及病人接受放射性治療後的預後不佳現象具有密切關聯
- MRE11透過上調化學趨化受體CXCR4的表現與其訊息傳遞來促進口腔癌細胞增生和轉移的能力
- MRE11的非核酸水解酶功能是促進上述口腔癌細胞增生和轉移現象的主因
- 預期MRE11可應用在臨床上成為口腔癌預後的重要標記與新的治療標的
【研究團隊】
汪硯雲副教授和袁行修教授的研究團隊長期致力於口腔癌之病因、致病機轉、治療及預防的研究及服務,團隊成員包括汪硯雲、陳玉昆、Steven Lo、紀宗辰、陳怡華、胡楚松、陳雅雯、江士昇、蔡芳瑜、劉旺達、李瑞年、謝雅晶、黃志仁、袁行修。
代表單位:
高雄醫學大學口腔醫學院牙醫學系及醫學院醫學研究所
團隊主要成員簡介:
1. 汪硯雲博士是高雄醫學大學口腔醫學院牙醫學系專任副教授,同時也是高雄醫學大學癌症研究中
心及高醫附院轉譯醫學研究中心的成員。 研究聯繫Email:wyy@kmu.edu.tw
2.袁行修博士是高雄醫學大學醫學院醫學研究所專任教授,同時也是高雄醫學大學癌症研究中心及高醫附院轉譯醫學研究中心的成員。 研究聯繫Email:yuanssf@kmu.edu.tw
【論文資訊】
論文出處:Oncogene. 2021, 40: 3510–3532.
全文下載:https://www.nature.com/articles/s41388-021-01698-5
New pathogenic mechanism and potential treatment of oral cancer
New pathogenic mechanism and potential treatment of oral cancer
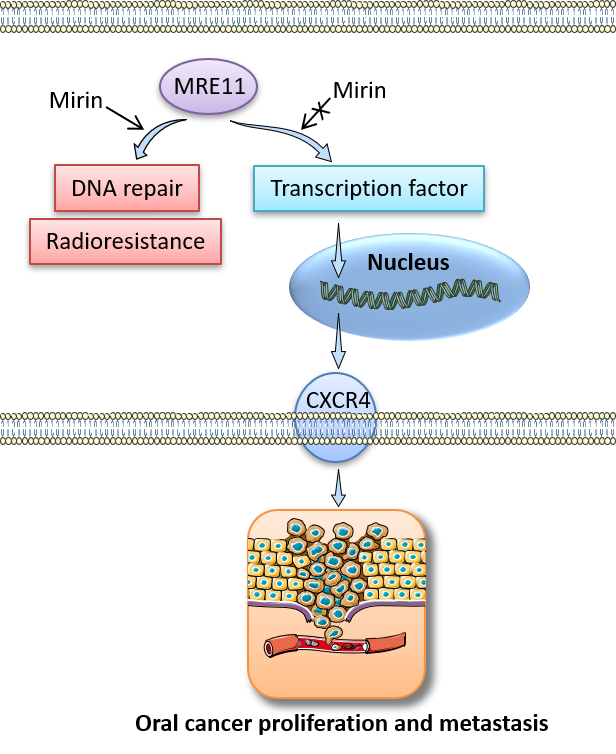
Oral cancer is one of the leading cause of cancer deaths in Taiwan, prevalently occurring in the male population. However, its biological mechanisms and specific therapeutic strategies remain to be explored. MRE11 is the core component of the RAD50/MRE11/NBS1 complex for repair of DNA double-strand-breaks, and has been known as a critical factor in solid tumor development through its nuclease-dependent activity. In this article, we found a novel role of MRE11 in oral cancer progression by its nuclease-independent function, and delineated its key downstream signaling through the chemoattractant receptor CXCR4 as follows. First, MRE11 expression in oral cancer samples was positively associated with the tumor size, cancer stage, and lymph node metastasis, and was predictive of poorer patient survival and radiotherapy resistance. In addition, MRE11 promoted oral cancer cell proliferation and migration/invasion ability, and the cancer-promoting effects of MRE11 were mediated by enhancing CXCR4 expression and its signaling. Notably, these phenomena were mitigated by using CXCR4 neutralizing antibodies in both in vitro and in vivo models. Moreover, inhibition of the nuclease-dependent activity of MRE11 by mirin reduced DNA repair of oral cancer cells; however, mirin treatment did not affect MRE11-promoted oral cancer cell proliferation and migration/invasion ability as described above, indicating that the nuclease-independent function of MRE11 underlies these pro-cancer effects. Collectively, MRE11 may serve as a clinical indicator and therapeutic target in oral cancer, displaying dual nuclease-dependent and independent roles that permit separate targeting of tumor vulnerabilities in oral cancer treatment. These findings will also facilitate future development of personalized medicine according to the differential expression of MRE11 in oral cancer patients.
Application and Highlights:
- High expression level of MRE11 in oral cancer tissues is associated with adverse oral cancer progression and treatment outcomes by radiotherapy
- MRE11 promotes oral cancer cell proliferation and migration/invasion ability through upregulation of chemoattractant receptor CXCR4 and its signaling
- These cancer-promoting effects result from the nuclease-independent function of MRE11
- MRE11 may have clinical benefits as a prognostic indicator and novel target for oral cancer treatment
Research Team Members:
Associate Professor Yen-Yun Wang and Professor Shyng-Shiou F. Yuan are dedicated to the translational research of the etiology, pathogenesis, treatment and prevention of oral cancer. Team members including Yen-Yun Wang, Yuk-Kwan Chen, Steven Lo, Tsung-Chen Chi, Yi-Hua Chen, Stephen Chu-Sung Hu, Ya-Wen Chen, Shih Sheng Jiang, Fang-Yu Tsai, Wangta Liu, Ruei-Nian Li, Ya-Ching Hsieh, Chih-Jen Huang & Shyng-Shiou F. Yuan
Representative Department:
Associate Professor Yen-Yun Wang:
School of Dentistry, College of Dental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
Professor Shyng-Shiou F. Yuan:
Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University
Introduction of Research Team:
The first author Yen-Yun Wang is a full-time associate professor in the School of Dentistry, Kaohsiung Medical University, and an appointed member of the Center for Cancer Research, Kaohsiung Medical University and Translational Research Center, Kaohsiung Medical University Hospital.
The corresponding author Shyng-Shiou F. Yuanis a full-time professor in the Graduate Institute of Medicine, Kaohsiung Medical University, and an appointed member of the Center for Cancer Research, Kaohsiung Medical University and Translational Research Center, Kaohsiung Medical University Hospital.
Contact Email:
Associate Professor Yen-Yun Wang: wyy@kmu.edu.tw
Professor Shyng-Shiou F. Yuan: yuanssf@kmu.edu.tw
Publication: Oncogene. 2021, 40: 3510–3532.
Full-Text Article: https://www.nature.com/articles/s41388-021-01698-5


