重大生物活性突破-透過環保途徑以酸改變合成所需化合物
重大生物活性突破-透過環保途徑以酸改變合成所需化合物
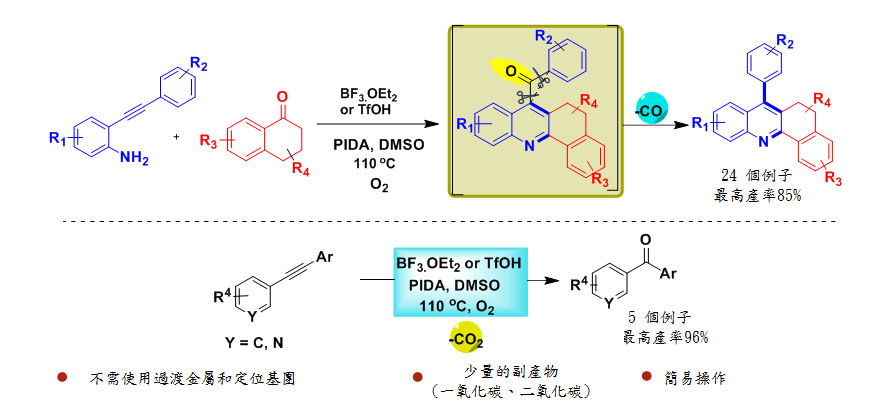
吖啶為重要的雜環芳香族化合物,具有多種生物活性,例如抗瘧疾,抗癌和抗白血病,因此,許多研究人員正在努力為吖啶衍生物尋找新的合成途徑。在此研究中,高雄醫學大學的研究人員開發了以酸促進分子內脫酮基偶合反應來合成吖啶。
以往脫酮基反應需在鹼性金屬錯合物、或酶、過氧化物和碘等條件下進行。這些方法大部分都是條件嚴苛或無選擇性,為了改善這個缺點,王志鉦教授團隊發現在無金屬條件下以酸促進酮分子內脫羰反應來合成吖啶衍生物。
這個方法條件溫和,對環境友善,不需過渡金屬催化,並可以使用易取得且低成本的酸在反應中取代貴金屬。重要的是,透過對甲氧基苯丙胺和氯化鈀來證實一氧化碳氣體的產生。此外,這個合成方法也可應用到簡單的芳香族內炔以產生二芳香基酮。
本篇為高雄醫學大學2020年3月份傑出論文得獎文章,代表作者為高雄醫學大學醫藥暨應用化學系教授王志鉦博士。
這項研究於2020年2月19日發表在《有機化學通訊》上,名為“吖啶衍生物的新合成方法”的研究通訊,可在以下網站在線獲取:https://pubs.acs.org/doi/pdf/10.1021/acs.orglett.0c00304 題為“吖啶衍生物的新合成方法”主要作者Ganesh Kumar Dhandabani,通訊作者包括王志鉦博士(高雄醫學大學醫藥暨應用化學系教授)。
媒體聯繫人:
高雄醫學大學醫藥暨應用化學系教授王志鉦博士
電話:+ 886-7-3121101(轉2275),電子郵件:jjwang@kmu.edu.tw
高雄醫學大學醫藥暨應用化學系博士後研究員 Ganesh Kumar Dhandabani
電話:+886-7-3121101(轉2275),電子郵件:ganechem@gmail.com
Imtramolecular decarbonylative coupling of unstrained ketones
Imtramolecular decarbonylative coupling of unstrained ketones
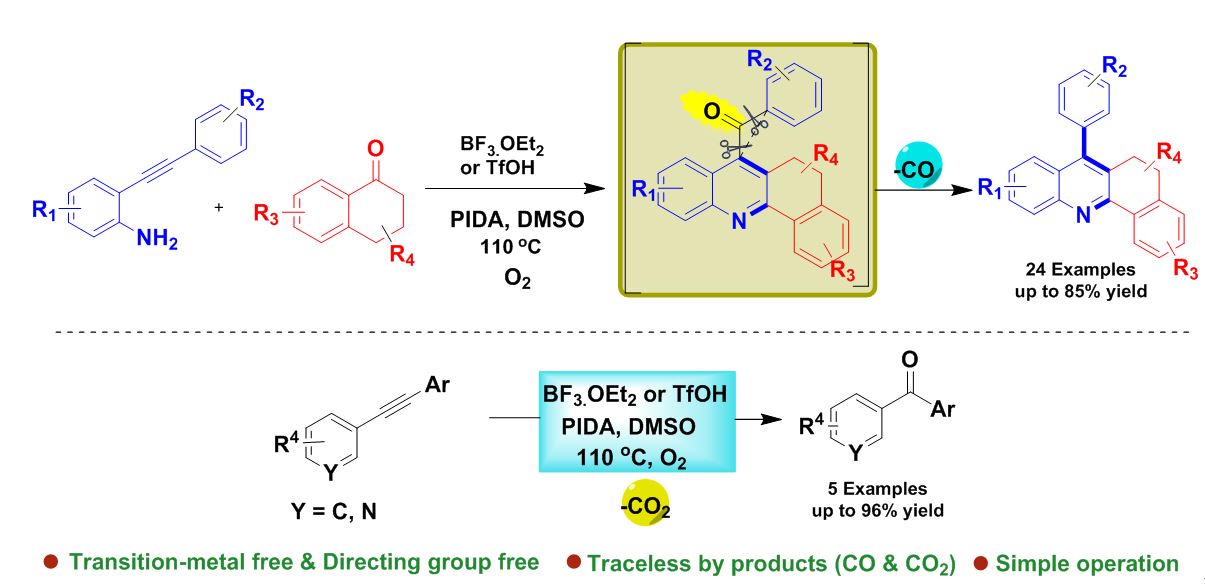
In this study, the researcher at Kaohsiung Medical University developed synthesis of acridines and diaryl ketones under acid promoted intramolecular decarbonylative coupling of unstrained ketone. Acridines are an important class of heteroaromatic compounds that have versatile biological properties, such as antimalarial, anticancer and antileukaemia. Therefore, several researchers are pursuing efforts to create new synthetic routes for acridine derivatives.
The success of intermolecular decarbonylation reactions of aldehyde, thioesters, amides, and anhydrides, intramolecular carbonyl group cleavage of ketone molecules remains challenging. This difficulty is likely due to the substantially inert nature of the C(Aryl)−C(O) bond and its hindered structure, making these motifs unsusceptible to the installation of a directing group. To develop a sustainable C−C bond activation method, basic metal complexes, enzymes, peroxides, and iodine source have been recently employed. The reported methods don’t have selective cleavage/activation of carbon bonds that accompanies transition metal-free C−C bond activation, especially for unstrained C−C bond cleavage. To overcome this drawback, in this study, the scientist Prof. Jeh-Jeng Wang and co-workers found that metal free acid promoted intramolecular decarbonylative of unstrained ketones for the synthesis of acridine derivatives.
The developed protocol is mild condition, environmentally friendly, transition-metal and directing group free, and can replace benchmark expensive noble metals in unstrained C−C bond activation reactions using abundant and low-cost acids. Importantly the liberation of CO gas confirmed by performing experiment with PMA-PdCl2.
This study was published online in Organic Letters on 19th February 2020 as a research communication entitled “Acid-Promoted Intramolecular Decarbonylative Coupling Reactions of Unstrained Ketones: A Modular Approach to Synthesis of Acridines and Diaryl Ketones” and is available online at https://pubs.acs.org/doi/pdf/10.1021/acs.orglett.0c00304.
The lead author of entitled “Acid-Promoted Intramolecular Decarbonylative Coupling Reactions of Unstrained Ketones: A Modular Approach to Synthesis of Acridines and Diaryl Ketones” Ganesh Kumar Dhandabani, Chia-Ling Shih, and corresponding author include Dr. Jeh-Jeng Wang (Professor, Kaohsiung Medical University, Department of Medicinal and Applied Chemistry). This article is award for Kaohsiung Medical University 2020 Monthly Excellent Paper Award in March.
Media Contact:
Dr. Jeh-Jeng Wang, Research fellow, Kaohsiung Medical University, Department of Medicinal and Applied Chemistry Tel: +886-7-3121101(Ext: 2275), E-mail: jjwang@kmu.edu.tw
Dr. Ganesh Kumar Dhandabani, Postdoctoral fellow, Kaohsiung Medical University, Department of Medicinal and Applied Chemistry Tel: +886-7-3121101(Ext: 2275), E-mail: ganechem@gmail.com
Chia-Ling Shih, Undergraduate student, Kaohsiung Medical University, Department of Medicinal and Applied Chemistry Tel: +886-7-3121101(Ext: 2275), E-mail: ymtc9801@gmail.com


