高濃度葡萄糖破壞肝臟細胞維生素 A衡定的發炎機制
高濃度葡萄糖破壞肝臟細胞維生素 A衡定的發炎機制
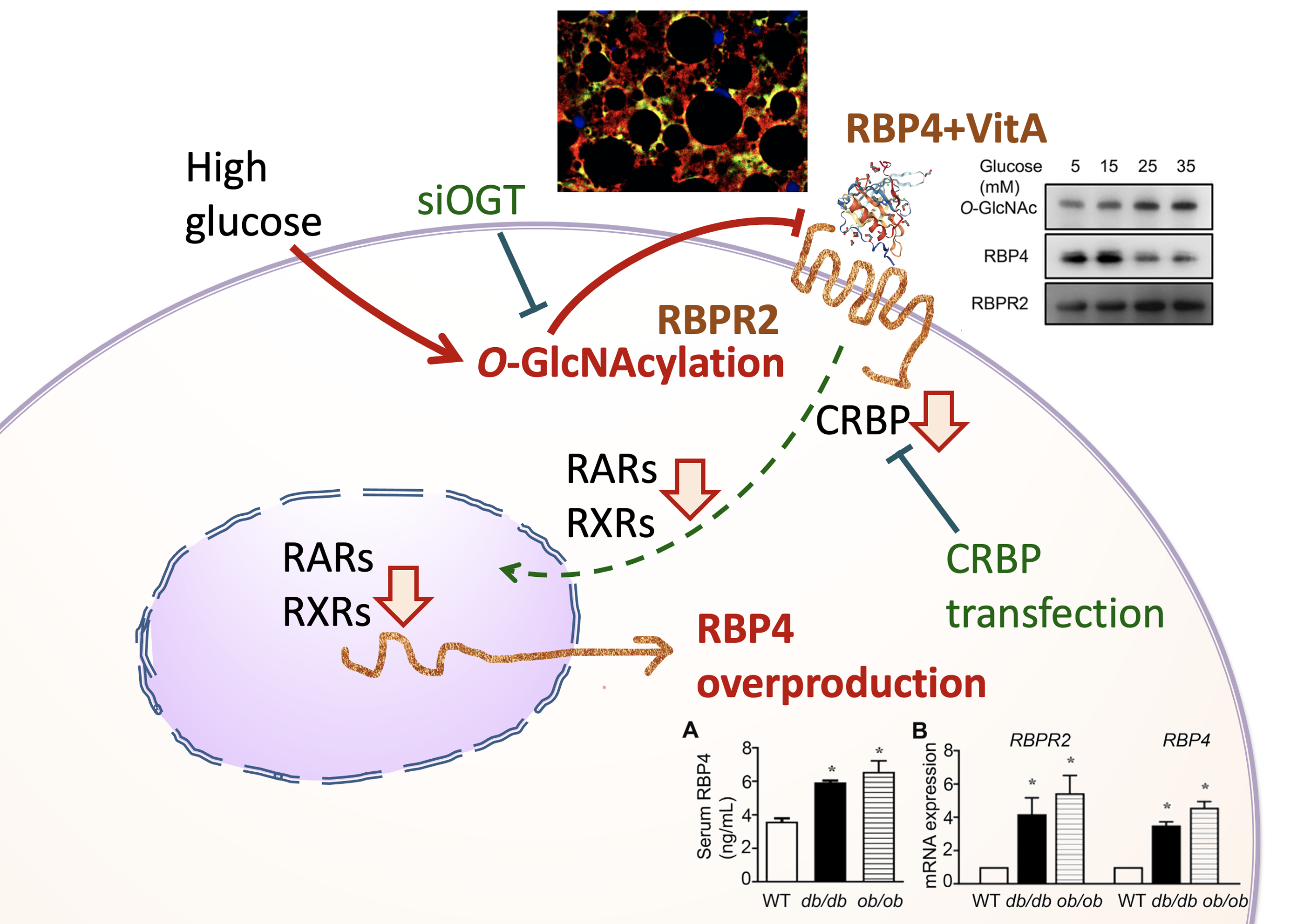
在代謝性疾病中,血液中的第四型視黃醇結合蛋白受體(Retinol-binding protein 4; RBP4)會表達增多,且與發炎反應有關。因此,本研究將進一步探討RBP4表達增多的細胞病理機制。我們假設糖尿病的病理特徵下,糖基化修飾(O-GlcNAcylation )會發生在肝臟的 RBP4 的細胞接受器(Retinol Binding Protein Receptor 2; RBPR2),導致肝臟細胞內retinol訊息被抑制,進而代償性地增加RBP4表現。
利用西方墨點法或免疫組織化學染色,我們檢查糖尿病老鼠(db/db, ob/ob mice)的肝臟retinol訊息傳遞相關蛋白質(RBPR2, CRBP1, LRAT, RALDH, RARα, RARγ, RXRα, RBP4)、糖基化修飾反應(GFAT, OGT, OGA)與發炎反應指標(inflammatory markers)表現量,也利用培養的肝臟細胞在高糖環境下驗證致病機轉。再者,利用免疫共沈澱與雙螢光染色,我們探討O-GlcNAc-modified RBPR2的蛋白質結合能力。最後,再以CRBP1 gene的轉染與O-Linked N-Acetylglucosamine (GlcNAc) Transferase (OGT) silencing 反向驗證上述的致病機轉。
實驗結果發現:在糖尿病老鼠會發現肝臟細胞的RBPR2被糖基化修飾,降低與RBP4結合能力,導致retinol cascade失衡。CRBP1基因轉染的高糖培養肝臟細胞反轉上述的失衡現象,降低 RBP4表現量與減少發炎。另外,OGT silencing 的高糖培養肝臟細胞也會降低retinol cascade失衡、降低RBP4表現量與減少發炎。
從本研究成果顯示:破壞 retinol cascade 與 RBP4 過度表現、肝臟發炎有關。而 RBPR2 的糖基化修飾是導致肝臟細胞維生素 A衡定破壞的原因。
圖形摘要
應用與亮點:
1. 解析高糖引起肝臟維生素A失去衡定的機制
2. 高糖引起肝臟細胞維生素A接受器(RBPR2)的糖基化反應與失能,造成細胞內維生素A不足,引起發炎反應與血液中載維生素A蛋白(RBP4)代償分泌增多,這與胰島素阻抗性增加有關。
3. 提供糖尿病、代謝症候群的創新治療策略,(1)抑制糖修飾酵素OGT;(2)增加肝細胞載維生素A蛋白(CRBP)。
【研究團隊】
團隊成員:柯良胤、辛錫璋 (請以超連結提供個人簡介網址;若無者免提供)
代表單位:醫學檢驗生物技術學系
團隊簡介:本研究是醫學檢驗生物技術學系柯良胤副教授、內分泌新陳代謝科辛錫璋教授、林昆德副教授、血脂生科研究中心詹華蓁等人跨領域研究合作之成果。研究經費由科技部與高雄醫學大學、高醫附設醫院共同支持。
研究聯繫Email:kly@gap.kmu.edu.tw
【論文資訊】
論文出處:期刊名. Metabolism年份月份2020 Dec; 期(卷): 頁數.113:154403.
全文下載:
https://www.sciencedirect.com/science/article/pii/S0026049520302675?via%3Dihub
High-glucose-induced O-GlcNAcylation disrupts the liver retinoid homeostasis
High-glucose-induced O-GlcNAcylation disrupts the liver retinoid homeostasis
Retinol-binding protein 4 (RBP4) is elevated and associated with inflammation in metabolic diseases. In this research, we aimed to investigate the detailed mechanism of RBP4 overproduction. We hypothesized O-linked GlcNAc modification targets RBPR2 and contributes to the disruption of retinol cascades in diabetic livers. Subsequently, the disruption of the retinol cascade induces RBP4 overproduction.
By western blot or immunohistochemistry, we examined RBPR2, CRBP1, LRAT, RALDH, RARα, RARγ, RXRα, RBP4, GFAT, OGT, OGA and inflammatory markers in livers of db/db and ob/ob mice and high glucose-cultured hepatocytes. By immunoprecipitation and dual fluorescence staining, we explored O-GlcNAc-modified RBPR2 and RBP4 binding activity on RBPR2. Transfection of the CRBP1 gene was done to verify whether a disrupted retinol cascade induces RBP4 overproduction. OGT silencing was done to investigate the association of O-GlcNAcylation with the disruption of the retinol cascade.
Disruption of retinol cascade, RBP4 overproduction, O-GlcNAcylation of RBPR2, decreased RBP4 binding activity on RBPR2 and inflammation were found in livers of db/db and ob/ob mice and high glucose-cultured hepatocytes. CRBP1 gene transfection reversed the suppression of the cellular retinol cascade and simultaneously attenuated the RBP4 overproduction and inflammation in high glucose-treated hepatocytes. The silencing of OGT reversed the disruption of the cellular retinol cascade, RBP4 overproduction and inflammation induced by high glucose in hepatocytes.
This study indicates that the cellular retinol cascade disruption is strongly associated with RBP4 overproduction and inflammation in diabetic livers. RBPR2 is one target for high glucose-mediated O-linked GlcNAc modification, which causes liver retinol dyshomeostasis.
Graphical Abstract

Application and Highlights:
1. Delineate mechanisms of high glucose-induced vitamin A dyshomeostasis in liver
2. In livers of diabetic mice, the retinol-binding protein receptor 2 (RBPR2) was O-GlcNAcylated and functionally impaired. O-GlcNAcylation of RBPR2 reduced the binding capability to retinol-binding protein 4 (RBP4) and cellular retinol signaling.
3. Silencing of O-GlcNAc transferase or CRBP1 transfection reversed high-glucose-induced retinol suppression, liver inflammation and lessened RBP4 overproduction.
Research Team Members: Liang-Yin Ke, Shyi-Jang Shin
Representative Department: Medical Laboratory Science and Biotechnology
Introduction of Research Team: The presented project was conducted by Associated Professor Liang-Yin Ke in department of Medical Laboratory Science and Biotechnology, Professor Shyi-Jang Shin, Kun-Der Lin in division of Endocrinology & Metabolism, and Hua-Chen Chan in Center for Lipid Biosciences.
Contact Email:
kly@gap.kmu.edu.tw
Publication: Metabolism. 2020 Dec;113:154403.
Full-Text Article: https://www.sciencedirect.com/science/article/pii/S0026049520302675?via%3Dihub

