長期精神壓力會造成慢性疼痛:揭開纖維肌痛症的病理機轉
長期精神壓力會造成慢性疼痛:揭開纖維肌痛症的病理機轉
您知道心理壓力除了造成焦慮、憂鬱等心理問題外,竟然也會造成生理上慢性全身疼痛嗎?
生活壓力在我們的日常生活中無所不在。除了經濟,家庭和工作上的壓力外,近期疫情爆發也造成民眾情緒和心理上不小的負擔。纖維肌痛症 (fibromyalgia) 是一種很常見卻又神秘的疼痛病。在成年人中,約冇2~6%的人罹患此病,是門診常見的疼痛疾患之一。病人常會抱怨有慢性廣泛性肌肉疼痛,並伴隨疲勞、失眠、焦慮和憂鬱等症狀。這些情形會嚴重影響病人的生活品質,甚至導致生活和工作大受影響。過去的臨床研究發現,日常生活精神壓力被認為會誘發或加重纖維肌痛症症狀。然而,壓力曝露與疾病發生間的因果關係在臨床研究上仍然難以認定,且醫學上對於該病的病生理機轉也仍然不清楚。由於纖維肌痛症和一般的肌肉發炎性疼痛疾病不同,抽血檢查通常呈現正常的結果,故病人常常於各科門診詳細檢查後而沒有發現明確病因。而目前對於症狀的處理,治療上仍以緩解症狀為主。
在和中研院共同合作的轉譯醫學博士學程中,高醫團隊的洪志憲醫和中研院生醫所陳志成研究員共組跨領域轉譯研究團隊。此外,高醫神經部賴秋蓮醫師,脂質科學暨老化研究中心陳珠璜教授,及屏科大蔡明憲博士也共同參與研究操作。經由建立動物的壓力模型,找出纖維肌痛症可能的致病機轉。研究發現,小鼠在經由反覆及間歇性心理壓力刺激後,會發生慢性廣泛性的疼痛,並伴隨疲勞及焦慮等行為變化,就如同臨床上纖維肌痛症的病情表垷。此外,研究也發現反覆壓力曝露會引發生物體內產生過量的氧化壓力, 而造成體內脂質的氧化性傷害,進而導致續發性的溶血卵磷脂LPC濃度上升。其中,過量的氧化脂質LPC16:0會活化肌肉組纖上常見的第三型酸感知離子通道(ASIC3),因而導致疼痛訊號的活化而造成慢性疼痛過敏感表現。氧化脂質LPC16:0除了表現在動物模型上,臨床上也可觀察到相對應的表現。藉由質譜儀的分析纖維肌痛症病人的血液檢體,在疼痛情況較嚴重的病人身上也可觀察到增加的LPC16:0表現。除上述機轉外,該研究也發現,藉由抑制氧化脂質LPC16:0的生成,藥物血小板活化因子乙醯水解酵素抑制劑(darapladib)在動物實驗上可有效的減輕壓力所造成的疼痛反應。這項發明未來或許可用於臨床作為纖維肌痛症的治療。這些研究成果,去年12月發表在國際風濕免疫科權威醫學期刊 Annals of the Rheumatic Diseases (IF:16.102)。
這個基礎科學與臨床醫學的跨領域團隊研究結果不僅提供了心理壓力可誘發慢性生理疼痛的直接實驗性證據,為過去長期的臨床觀察性研究結果提供了病生理機轉的合理解釋,也為未來纖維肌痛症的研究提供了良好的轉譯研究平台。藉由研究這種壓力所引發的疼痛的機轉,可以幫助釐清纖維肌痛症可能的致病原因,以幫助發展更好的診斷和治療方式。
圖形摘要
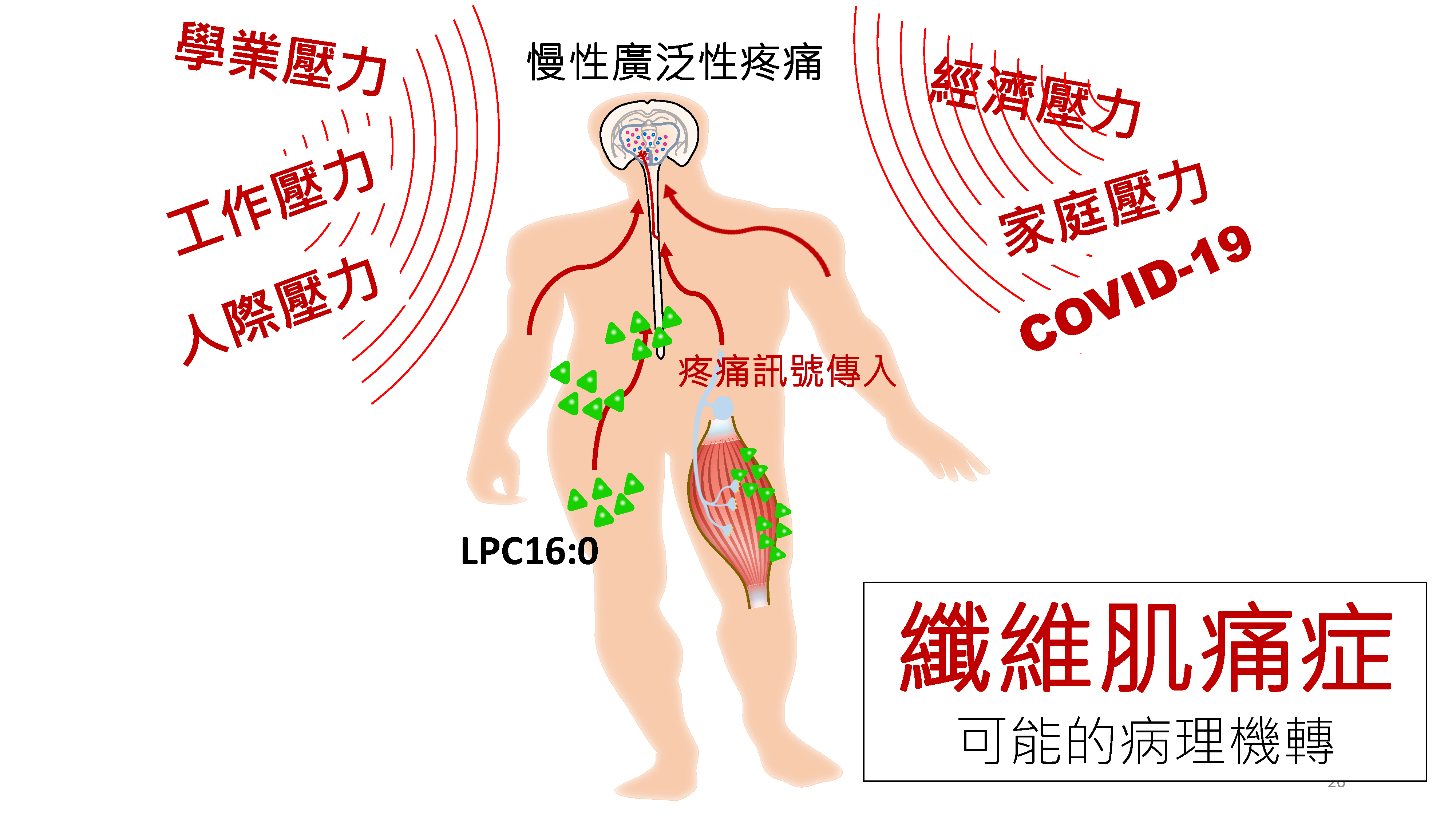
應用與亮點:
1.找到心理壓力和纖維肌痛症發病的可能因果關係。
2.發現藉由調控致痛性脂質LPC16:0,找到減緩或預防纖維肌痛症的可能有效治療。
【研究團隊】
洪志憲醫師,陳志成教授,賴秋蓮教授,陳珠璜教授,蔡明憲博士
代表單位:高雄醫學大學附設中和紀念醫院神經部
團隊簡介:洪志憲醫師,目前任職於高醫附院神經部。除了臨床工作,洪醫師也完成基礎及轉譯醫學學程的進修,進行疾病機轉及動物模型研究。陳志成教授,目前任職於中研院生醫所研究員兼副所長。陳教授多年來致力於討引起慢性肌肉酸痛的分子機制,以及臨床疾病“痠”與”痛”的研究。
研究聯繫Email:jasonhung0701@gmail.com (personal)
【論文資訊】
論文出處:Annals of the Rheumatic Diseases 2020 Dec;79(12):1644-1656..
全文下載:Activation of acid-sensing ion channel 3 by lysophosphatidylcholine 16:0 mediates psychological stress-induced fibromyalgia-like pain | Annals of the Rheumatic Diseases (bmj.com)
Psychological stress triggers physical pain – revealing the mysterious pathophysiology of fibromyalgia
Psychological stress triggers physical pain – revealing the mysterious pathophysiology of fibromyalgia
As is well known, psychosocial stress can result in psychiatric disorders, such as depression and anxiety. But do you know that psychological stress can also cause chronic physical pain?
In the current pandemic of COVID-19, psychological stress is pervasive and inevitable globally for people. Psychosocial stress can not only precipitate psychiatric illness but also various somatic pain disorders. Fibromyalgia is a very common but mysterious pain disorder, and the prevalence is up to 2~6 % of the general adult population. This illness is characterized by chronic widespread muscular pain. Fatigue, anxiety and depression are the common comorbidities. Mounting evidence of clinical observational studies suggests that daily psychological stressors trigger or aggravate pain in fibromyalgia. However, the causation of stress and disease development remains poorly defined by clinical investigations. Also, the underlying mechanism remains largely unknown. Unlike other inflammatory pain disorders, laboratory tests in these patients commonly show non-specific findings. Accordingly, patients with this pain disorder may fail to have a definite diagnosis despite intensive examination. Also, current therapeutic approaches focus on symptomatic relief rather than curative treatment.
To this end, neurologist Chih-Hsien Hung in Kaohsiung Medical University hospital and neuroscientist Chih-Cheng Chen in Academia Sinica (Taiwan) formed an interdisciplinary team of translational research to tackle fibromyalgia. By establishing a novel translational model and investigating its mechanism, Dr. Hung and colleagues identified a possible pathophysiological mechanism of fibromyalgia. Using non-painful sound stimuli as psychological stressors, the group found that repeated and intermittent exposure to psychological stressors triggered long lasting non-inflammatory pain behaviors in mice. The stressed mice also had fatigue and anxiety-like behaviors, as observed in clinical conditions of fibromyalgia. Additionally, the study found that repeated psychological stress induced excessive oxidative stress in animals, which thus caused lipid oxidization and subsequent upregulation of lysophosphatidylcholine (LPC) 16:0. Excessive LPC16:0 actives acid-sensing ion channel 3 on the muscle tissue to trigger nociceptive signaling and cause chronic hypersensitivity. Excessive oxidative stress and LPC16:0 expression was observed in the translational model and also in clinical investigation of fibromyalgia. Clinical lipidomic profiling of blood samples showed excessive LPC16:0 expression in fibromyalgia cases, and LPC16:0 expression was correlated with pain symptoms in patients with high oxidative stress and disease severity. Furthermore, darapladib (a platelet-activating factor-acetylhydrolase inhibitor; previously designed for anti-atherogenic purposes) effectively alleviated the stress-induced hypersensitivity by inhibiting LPC16:0 synthesis in mice, which might guide a new therapeutic approach for fibromyalgia. The study results have been published in the Annals of the Rheumatic Diseases (December, 2020).
Graphical Abstract
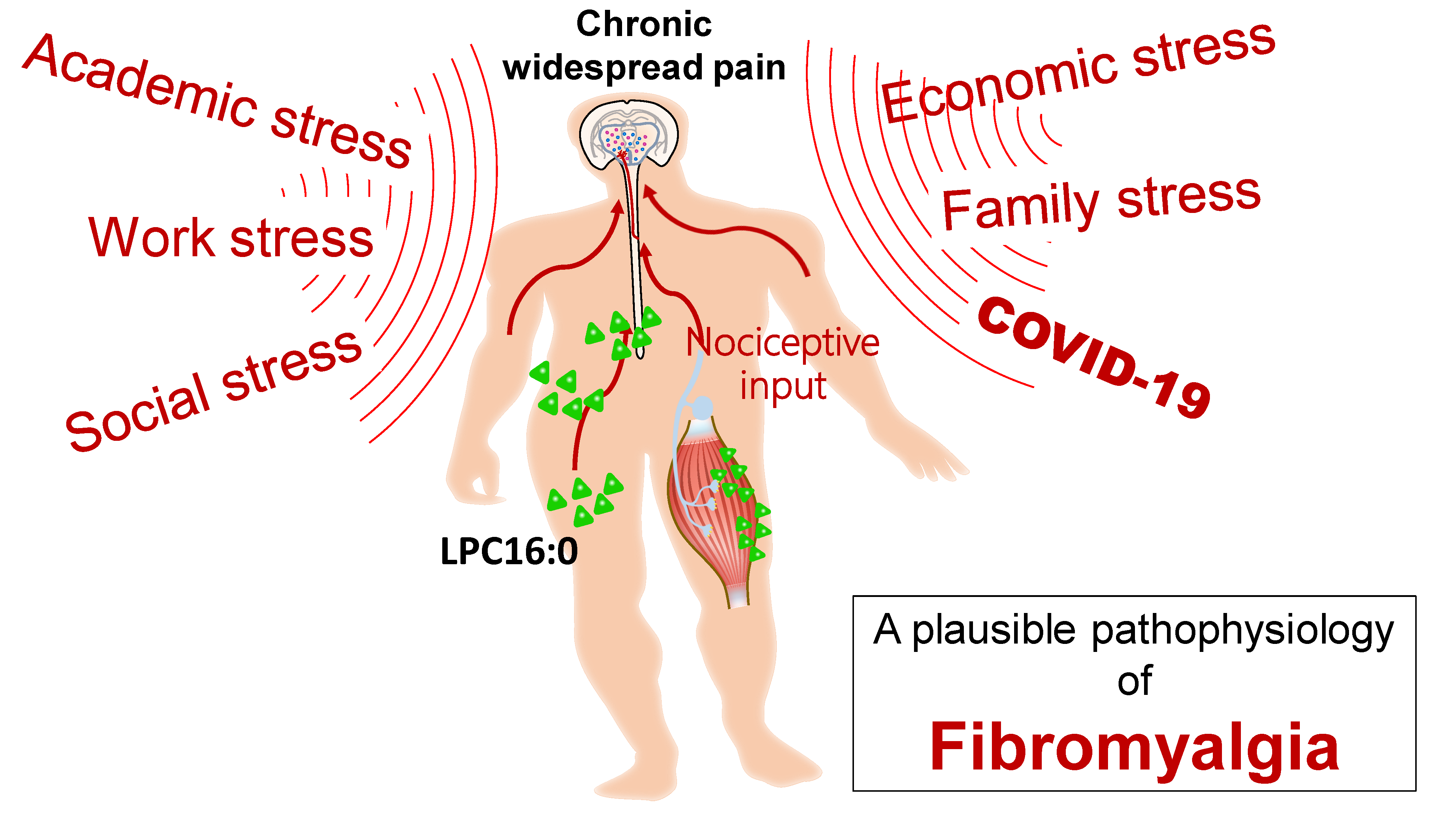
Application and Highlights:
1.This research identified a plausible causative mechanism of psychological stress exposure and
fibromyalgia development.
2.The research found a possible therapeutic approach for fibromyalgia by modifying the algesic oxidized
lipid LPC16:0.
Research Team Members: Dr. Hung Chih-Hsien, Prof. Chih-Cheng Chen, Prof. Chiu-Lian Lai, Prof. Chu-Huang Chen and Dr. Ming-Hsien Tsai.
Representative Department: Department of Neurology, Kaohsiung Medical University Hospital
Introduction of Research Team: Dr. Hung is currently an attending physician of Kaohsiung Medical University Hospital. Professor Chih-Cheng Chen is deputy director of Institute of Biomedical Sciences of Academia Sinica at Taipei, Taiwan. He is also director of Taiwan Mouse Clinic.
Contact Email: jasonhung0701@gmail.com
Publication: Annals of the Rheumatic Diseases 2020 Dec;79(12):1644-1656.
Full-Text Article: Activation of acid-sensing ion channel 3 by lysophosphatidylcholine 16:0 mediates psychological stress-induced fibromyalgia-like pain | Annals of the Rheumatic Diseases (bmj.com)


