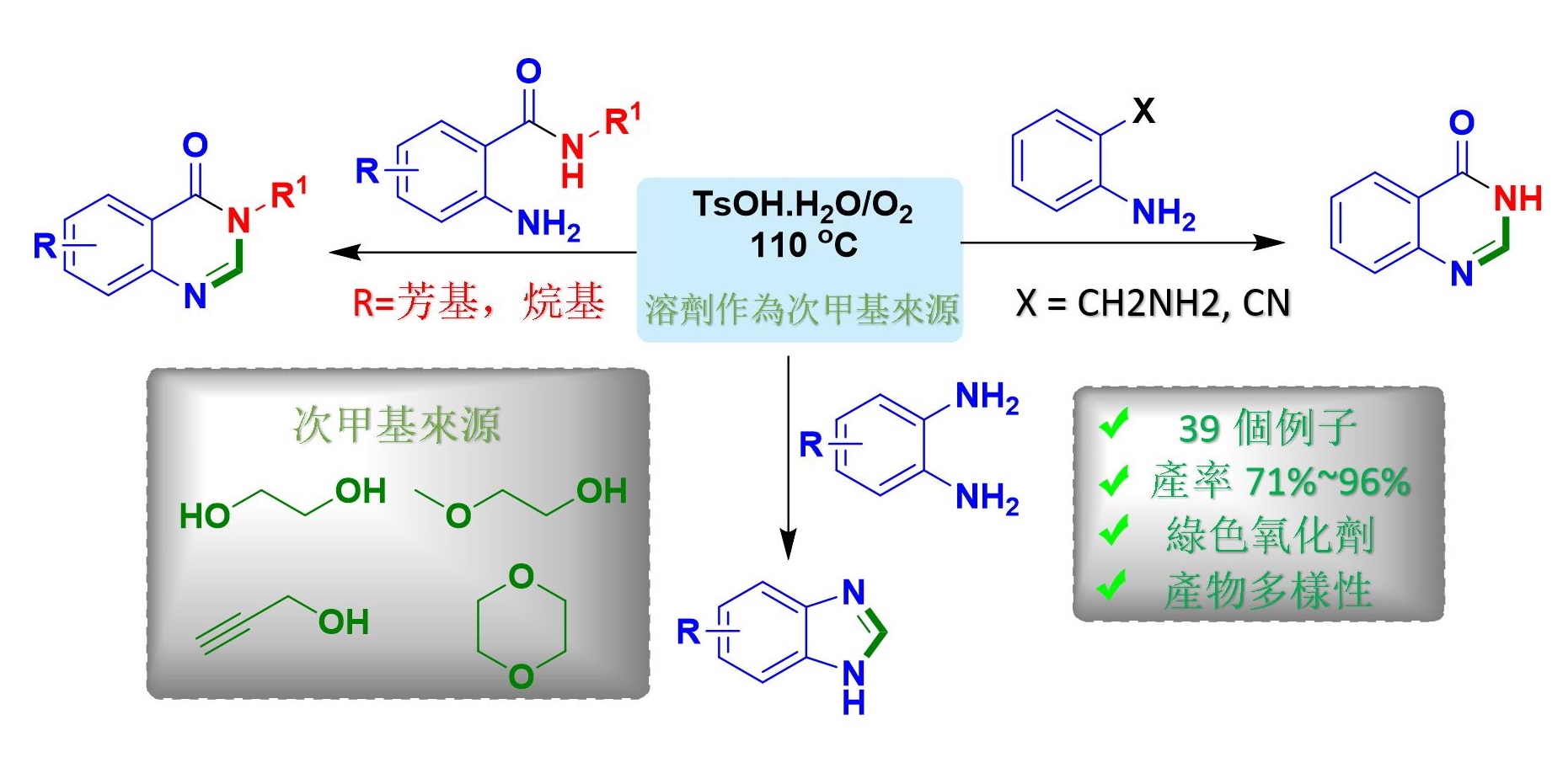
新型環保的次甲基源可作為合成雜環化合物
新型環保的次甲基源可作為合成雜環化合物
在最近的研究中,高雄醫學大學的研究人員開發了一種新的化學方法,利用醇類和醚類作為次甲基 –CH 的來源,用於合成喹唑啉酮和苯並咪唑衍生物,這類化合物通常具有藥理特性,如: 抗微生物,抗驚厥,鎮靜,降壓,抗抑鬱,抗炎和抗過敏特性藥物,因此值得推薦應用本方法來合成這類衍生物。
由於前人報導合成喹唑啉的方法受限於產物的範圍、需使用強酸性條件、昂貴的金屬催化劑、爆炸性過氧化物以及長時間反應。 因此,為了克服這些缺陷,王志鉦教授團隊最新研究中發現,在友善環境下以氧氣作為氧化劑,使用醚或醇類為次甲基來源可合成異核芳香族化合物。
此外,這個研究團隊也發現,利用本方法可合成具有生物活性的乙酰膽鹼酮、二咪唑、蕓苔芸香鹼、(±)依夫二胺的前驅物。 隨著乙二醇作為“次甲基”來源的成功,研究團隊進一步確定其他醇和醚亦可作為次甲基來源,此由重氫標記研究證實了這一結果。
這項研究於2019年2月1日發表在《綠色化學》上,名為 ”在無金屬和無過氧化物的條件下,永續次甲基源合成雜環”,可在以下網站在線獲取:https://pubs.rsc.org/en/content/articlepdf/2019/gc/c8gc03839b. (Green Chem., 2019, 21, 979–985)
本論文共同第一作者為Vishal Suresh Kudale和Gopal Chandru Senadi博士,通訊作者為王志鉦教授(高雄醫學大學醫藥暨應用化學系教授 )。
高雄醫學大學醫藥暨應用化學系教授王志鉦教授
電話:+ 886-7-3121101(轉2275),電子郵件:jjwang@kmu.edu.tw
高雄醫學大學醫藥暨應用化學系博士生Vishal Suresh Kudale
電話:+886-7-3121101(轉2275),電子郵件:vishalkudale90@gmail.com
高雄醫學大學醫藥暨應用化學系博士後研究員Gopal Chandru Senadi
電話:+ 886-7-3121101(轉:2275),電子郵件:sgchand@gmail.com
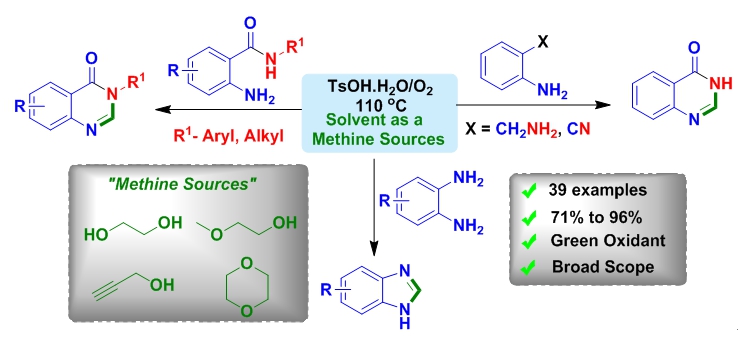
Novel eco-friendly methine sources can synthesize useful heterocycles
Novel eco-friendly methine sources can synthesize useful heterocycles
In the recent study, the researchers at Kaohsiung Medical University have developed a new chemical method to introduce alcohols and ethers as carbon source (“-CH”) for the synthesis of quinazolinone and benzimidazole derivatives which are highly recommended due to their pharmacological properties, e.g. anti-microbial, anticonvulsant, sedative, hypotensive, anti-depressant, antiinflammatory, and anti-allergy properties.
Previous approaches for the synthesis quinazolines have suffered from limited substrate scopes and strong acidic conditions, expensive metal catalysts, explosive peroxides and long reaction times. So to overcome these drawbacks, in the latest study, Prof. Jeh-Jeng Wang and co-workers found that ethers and alcohols can be used as carbon synthon for the synthesis of heteroarenes in the presence of environmentally benign O2 as an oxidant.
Furthermore, Prof. Wang’s group demonstrated the applicability of this method by synthesizing biologically active echinozolinone, dimedazole, and common precursor of rutaecarpine and (±) evodiamine. With the success of ethylene glycol as a “methine” source, the researchers also identified other alcohols and ethers as a carbon synthons for the synthesis of quinazolinone derivatives. This result was confirmed by Deuterium labelling studies.
This study was published online in Green Chemistry on 1st February 2019 as a research communication entitled “Sustainable methine sources for the synthesis of heterocycles under metal- and peroxide-free conditions” and is available online at https://pubs.rsc.org/en/content/articlepdf/2019/gc/c8gc03839b(Green Chem., 2019, 21, 979–985)
The lead authors of the work entitled “Sustainable methine sources for the synthesis of heterocycles under metal- and peroxide-free conditions” are Vishal Suresh Kudale and Gopal Chandru Senadi, corresponding author include Dr. Jeh-Jeng Wang (Professor, Kaohsiung Medical University, Department of Medicinal and Applied Chemistry, Taiwan).
Media Contact:
Prof. Jeh-Jeng Wang, Kaohsiung Medical University, Department of Medicinal and Applied Chemistry Tel: +886-7-3121101(Ext: 2275), E-mail: jjwang@kmu.edu.tw
Vishal Suresh Kudale, Doctoral fellow, Kaohsiung Medical University, Department of Medicinal and Applied Chemistry Tel: +886-7-3121101(Ext: 2275), E-mail: vishalkudale90@gmail.com
Gopal Chandru Senadi, Postdoctoral fellow, Kaohsiung Medical University, Department of Medicinal and Applied Chemistry Tel: +886-7-3121101(Ext: 2275), E-mail: sgchand@gmail.com


