藍光誘導分子化學鍵斷裂、重組以合成具藥物活性結構之化合物
藍光誘導分子化學鍵斷裂、重組以合成具藥物活性結構之化合物
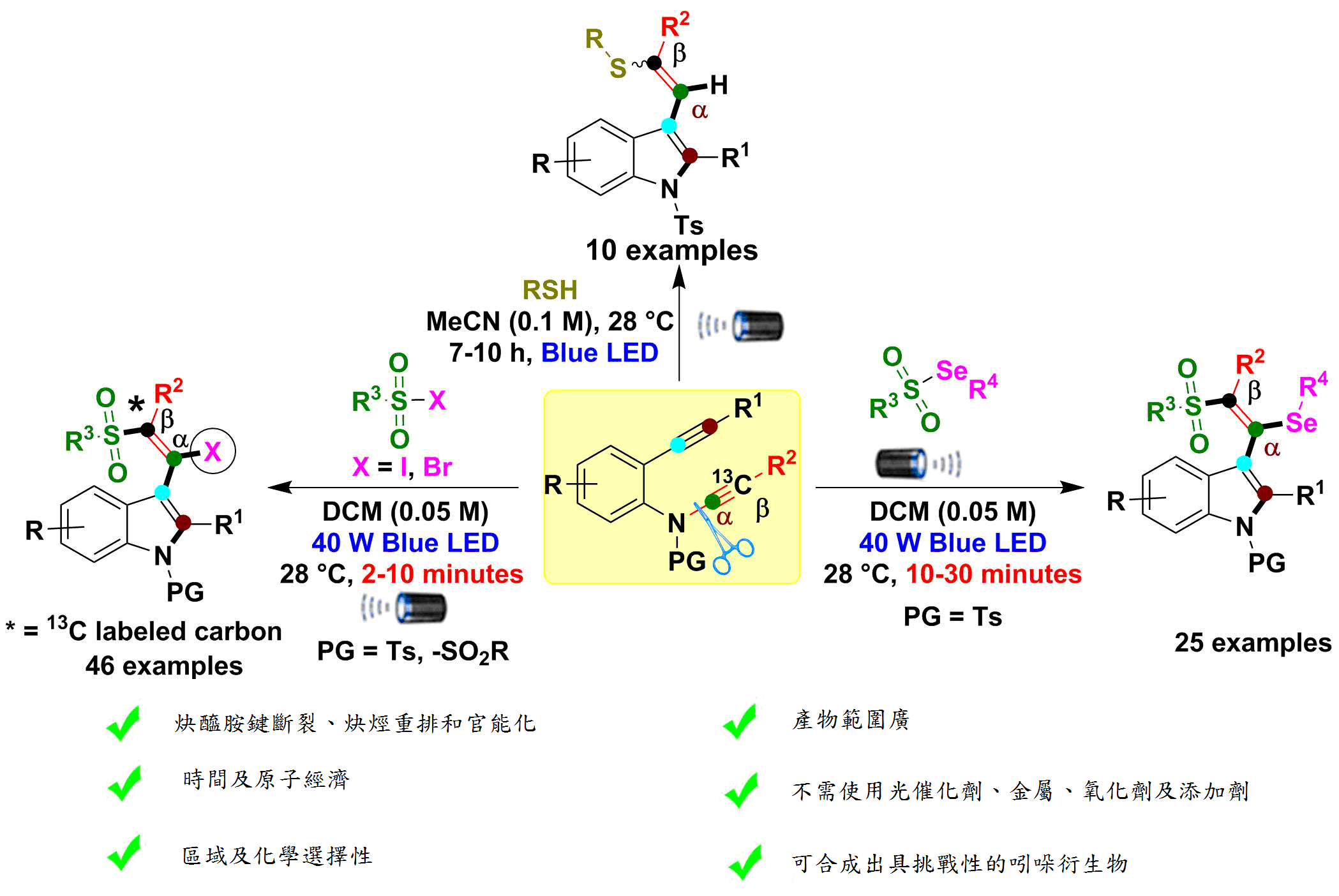
LED光源近年來已漸漸被應用到有機合成上,因其使用方便、環保又有效率。高醫研究團隊利用藍光LED誘導炔醯胺選擇性自由基C(sp)-N鍵裂解、重排和官能化,本反應時間僅2-10分鐘即合成具有含硫屬元素取代的吲哚衍生物。吲哚結構出現在許多上市藥物、材料化學和許多其他領域中。以往合成吲哚需要使用昂貴的金屬、光催化劑、氧化劑、較長的反應時間、有害的廢物產生和缺乏原子經濟性。更重要的是,直到現在,選擇性分子間自由基引發的炔醯胺鍵裂變和結構重排仍然是炔醯胺化學中未解決的挑戰。
為了克服上述缺點,高醫大王志鉦教授和Mohana Reddy Mutra博士後研究員在這項研究中,以藍色LED照射誘導炔醯胺 C(sp)-N 鍵斷裂、炔烴重排和官能化與各種自由基起始物來產生新的吲哚衍生物。所開發的方案是環保的,不需使用光催化劑、金屬、氧化劑、添加劑,反應條件溫和且只需幾分鐘即可完成。這類化合物的藥物活性測試正在進行中。
圖形摘要:
應用與亮點:
1. 成功合成具有挑戰性的硫屬元素取代吲哚衍生物。
2. 選擇性自由基介導的炔醯胺 C(sp)-N 鍵斷裂、炔烴重排和官能化。
3. 無需添加昂貴的金屬和催化劑即可在極短的反應時間內進行。
4. 產物範圍廣,為對環境友好、具有區域和化學選擇性、步驟和原子經濟的反應。
【研究團隊】
團隊成員:王志鉦教授
Mohana Reddy Mutra博士後研究員
代表單位:高雄醫學大學醫藥暨應用化學系
團隊簡介:高雄醫學大學醫藥暨應用化學系 王志鉦 教授
電話:+ 886-7-3121101(轉2275),電子郵件:jjwang@kmu.edu.tw
高雄醫學大學醫藥暨應用化學系 Mohana Reddy Mutra 博士後研究員
電話:+886-7-3121101(轉2275),電子郵件:mohan.mohan2060@gmail.com
【論文資訊】
論文出處:Nat. Commun. 2022, 13, 2345.
全文下載: https://www.nature.com/articles/s41467-022-30001-7.
Photoinduced ynamide structural reshuffling and functionalization
Photoinduced ynamide structural reshuffling and functionalization
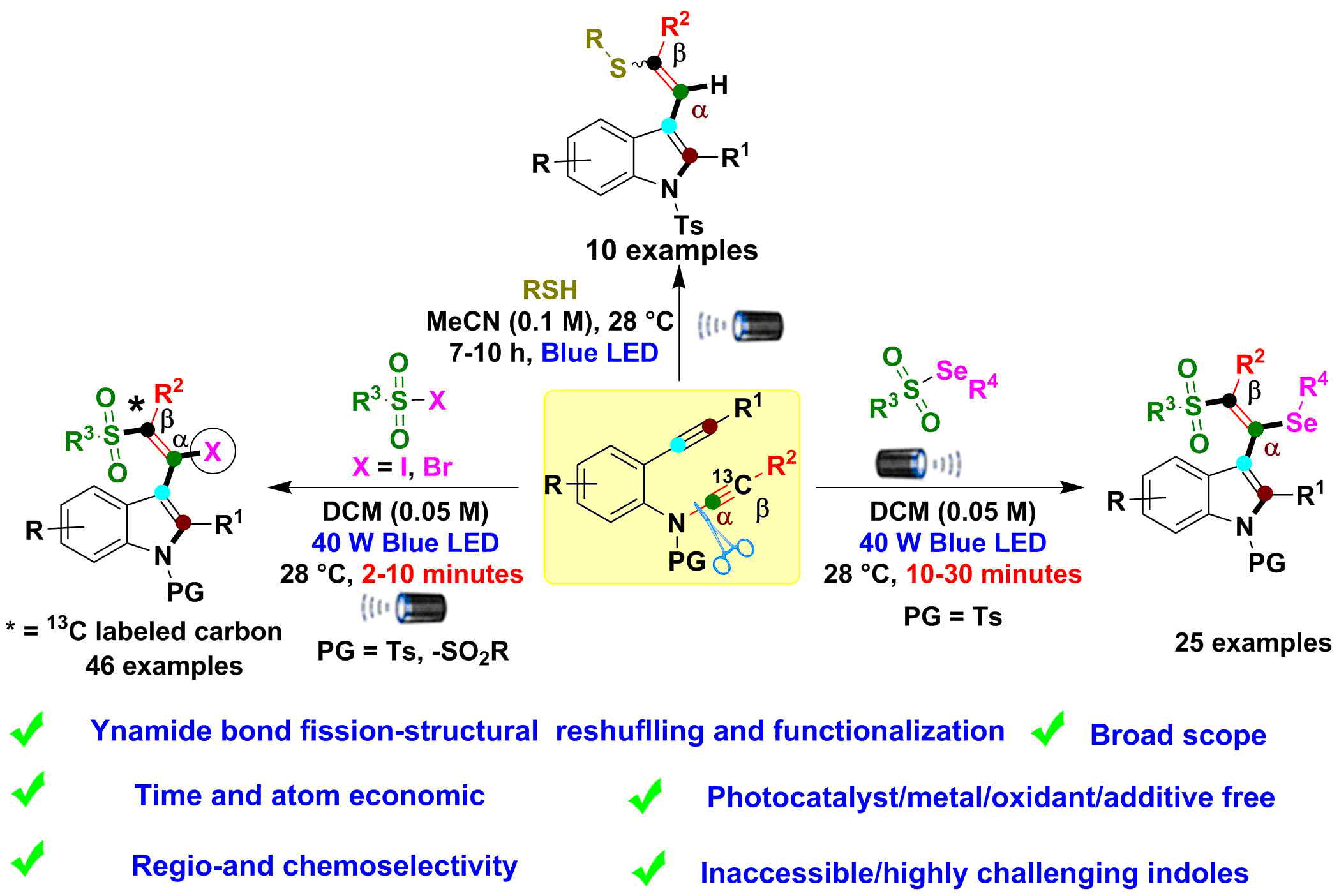
In this study, the researchers at Kaohsiung Medical University developed blue LED-induced selective radical-mediated ynamide C(sp)-N bond cleavage, rearrangement and functionalization by using 2-alkynyl-ynamides with various radical precursors for the synthesis of challenging chalcogen-substituted indole derivatives with excellent step/atom economy.
Indoles are favorable structural motifs that appear in numerous marketed drugs, the pharmaceutical industry, drug discovery, material chemistry and numerous other fields, including recent therapeutic leads. Thus, the construction of a new indole ring system was a goal for altering the native indole cores, thus enabling access to chalcogen-substituted indoles as potential building blocks for drug discovery in the future. Most importantly, the available active C–I bond in the products can be used to achieve structural modification of bioactive compounds, drugs and drug leads, as well as natural products.
The literature methods for new indole molecule synthesis require expensive metals/photocatalysts, oxidants, longer reaction times, harmful waste production, and a lack of atom economy. Importantly, selective intermolecular radical-initiated ynamide bond fission and structural rearrangement have remained unanswered challenges in ynamide chemistry until now.
To overcome the above drawback, in this study, the scientist Prof. Jeh-Jeng Wang and Dr. Mohana Reddy Mutra found a simple blue LED light mediated ynamide C(sp)–N bond cleavage, alkyne rearrangement and functionalization to produce new indole derivatives with various radical precursors. The developed protocol is environmentally-friendly, photocatalysts/metals/ oxidants/additives-free, mild condition, and the reaction time is very short (few minutes). Control experiments and 13C-labeling experiments proved, sulfone/thiol radicals contribute to ynamide structural reshuffling processes via a radical pathway.
Graphical Abstract:
Application and Highlights:
1. Successfully synthesized challenging chalcogen-substituted indole derivatives.
2. Selective radical-mediated ynamide C(sp)–N bond cleavage, alkyne rearrangement and functionalization.
3. The photocatalytic reaction can be carried out without adding expensive metals and catalysts in very short reaction time.
4. Broad scope, environmentally friendly, regio- and chemoselective, step and atom economy.
Research Team Members:
Dr. Jeh-Jeng Wang and Mohana Reddy Mutra
Representative Department: Department of Medicinal and Applied Chemistry, Kaohsiung Medical University
Introduction of Research Team:
Dr. Jeh-Jeng Wang, Research fellow, Kaohsiung Medical University, Department of Medicinal and Applied Chemistry Tel: +886-7-3121101(Ext: 2275), E-mail: jjwang@kmu.edu.tw
Dr. Mohana Reddy Mutra, Postdoctoral fellow, Kaohsiung Medical University, Department of Medicinal and Applied Chemistry Tel: +886-7-3121101(Ext: 2275), E-mail: mohan.mohan2060@gmail.com
Contact Email: jjwang@kmu.edu.tw
Publication: Nat. Commun. 2022, 13, 2345.
Full-Text Article:
https://www.nature.com/articles/s41467-022-30001-7.


