近紅外線照射可以抑制皮膚發炎
近紅外線照射可以抑制皮膚發炎
光生物調節 (Photobiomodulation; PBM) 是一種利用可見光或近紅外光(NIR;波長700–1100 nm)來刺激細胞功能的治療方法,廣泛的應用在醫學保健領域,在傷口癒合、緩解疼痛和減少發炎方面顯示出顯著效果。
在生物組織中,光的吸收和穿透力取決於光的波長與組織成分間的相互作用,簡單的說,600奈米(nm)以下波長光會被血紅蛋白等組織成分吸收,而波長大於1100 nm的光(中/遠紅外光)會被水吸收。700-1100 nm的位於組織光學吸收空窗 (tissue optical window),因此近紅光對組織的穿透力是最大的,近紅外光可穿透皮膚高達5毫米 (mm),可深及皮下組織甚至表淺的骨組織,以調節深層且複雜的細胞交互作用,包含免疫反應。
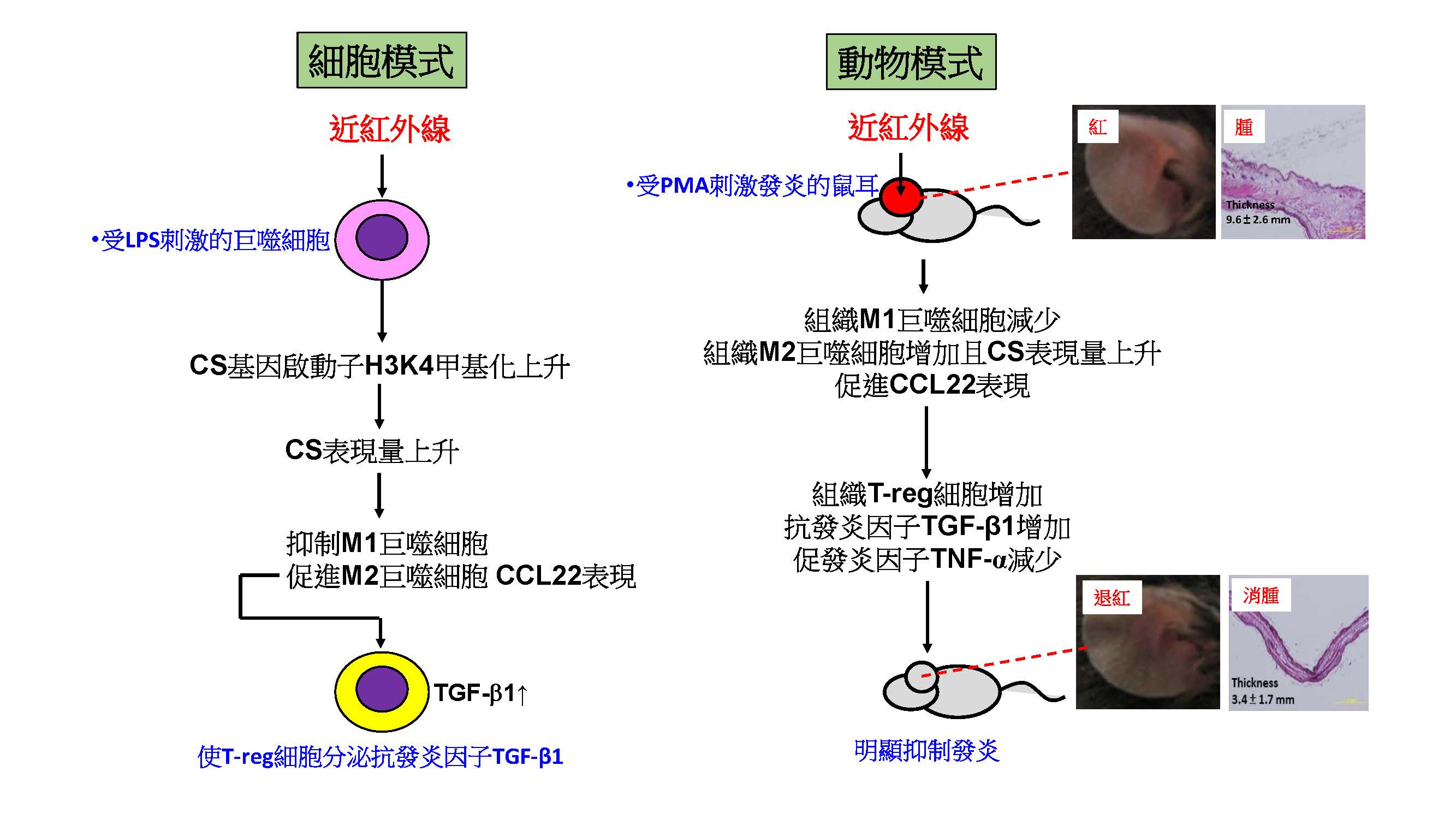
巨噬細胞會通過其 M1/M2 極化的改變來指導免疫反應: M1主導發炎,M2主導抗發炎。在本篇研究中,我們闡明了近紅外光誘導巨噬細胞的分子機制。經由蛋白質體分析,我們發現近紅外光照射增強了線粒體呼吸鏈中檸檬酸合酶(CS)的表達,而不是由熱觸發的。這種改變是由近紅外光引起的表觀基因調控所致 (epigenetic regulation; 一種至少可維持數周的基因開/關機制)。這導致發炎性的M1巨噬細胞走向M2極化,最終導致T細胞釋放 TGF-β1,而TGF-β1是一種抗發炎及促進修補的代表性蛋白,因此推論近紅外線可能具有抗發炎的功效。
這些推論在小鼠實驗中得到了驗證,在誘發小鼠皮膚發炎症模式中,我們證實近紅外照射 (能量60 J/cm2,照射一次) 會減少發炎性的M1細胞數量、減少發炎因子 TNF-α,和增加抗發炎CCL22/TGF-β1。經近紅外線照射的小鼠,其紅腫等發炎症狀,可回復至接近沒有發炎時的狀態。
圖形摘要
應用與亮點:
1.發現近紅外線影響免疫的核心機制 (表觀基因修飾)
2.釐清近紅外線抗發炎的原理 (釋放 TGF-β1)
3.提供近紅外線臨床應用的理論依據
【研究團隊】
團隊成員:
廖偉廷教授、余幸司教授
代表單位:高雄醫學大學生物科技學系
團隊簡介:廖偉廷老師的主要研究方向是巨噬細胞在癌症和發炎疾病中的調控,並在體外以組織微環境模式重建這些調控,以評估可能的防治策略。
研究聯繫Email: wtliao@kmu.edu.tw
【論文資訊】
論文出處:J Invest Dermatol. 2021 Aug; 141(8): 2056-2066.e10
全文下載:https://www.sciencedirect.com/science/article/pii/S0022202X21001573
Near-infrared irradiation shows significant anti-inflammatory effects of via M2 macrophage polarization
Near-infrared irradiation shows significant anti-inflammatory effects of via M2 macrophage polarization
Photobiomodulation (PBM) is a therapeutic method that utilizes visible light or near-infrared light (NIR; wavelength 700-1100 nm) to stimulate cellular functions. It has been widely applied in the medical and healthcare fields, showing significant effects in wound healing, pain relief, and inflammation reduction.
In biological tissues, the absorption and penetration of light depend on the wavelength and interactions with tissue components. In simple terms, wavelengths below 600 nanometers (nm) are absorbed by tissue components like hemoglobin, while wavelengths greater than 1100 nm (mid/far-infrared light) are absorbed by water. The range of 700-1100 nm falls within the tissue optical window, making near-infrared light have the maximum penetration into tissues. Near-infrared light can penetrate the skin up to 5 millimeters (mm), reaching deep subcutaneous tissues and even superficial bone tissues, allowing it to modulate deep and intricate cellular interactions, including immune responses.
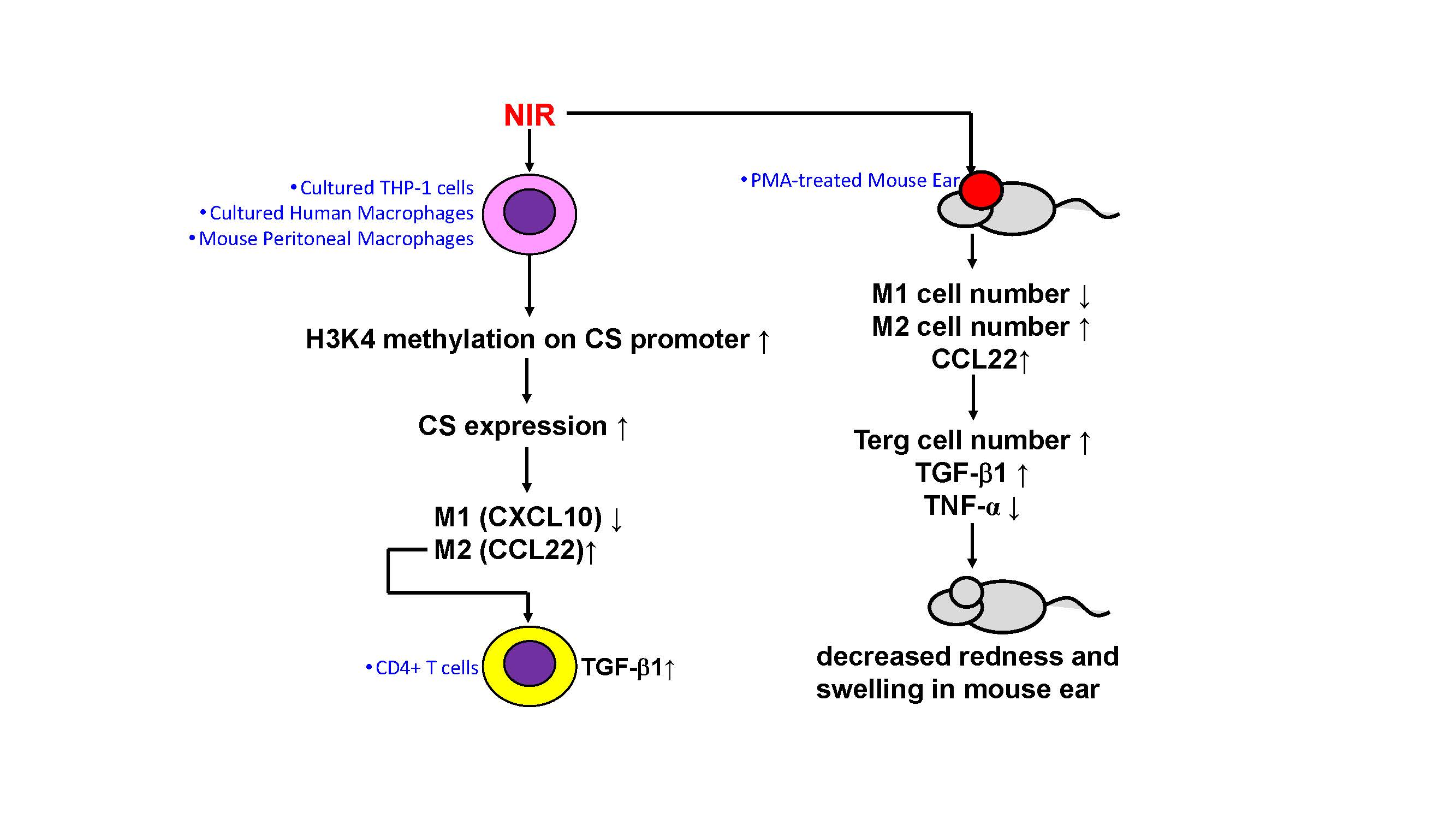
Macrophages guide immune responses through their M1/M2 polarization, where M1 dominates inflammation and M2 dominates anti-inflammation. In this study, we elucidated the molecular mechanisms of near-infrared light-induced macrophage polarization. Through proteomic analysis, we found that near-infrared light exposure enhanced the expression of citrate synthase (CS) in the mitochondrial respiratory chain, and this change was not thermally induced but caused by epigenetic regulation (a gene on/off mechanism that can be maintained for weeks). This led to the transition of inflammatory M1 macrophages towards M2 polarization, ultimately resulting in the release of TGF-β1 by T cells. TGF-β1 is a representative protein with anti-inflammatory and repair-promoting properties, suggesting that near-infrared light may have anti-inflammatory effects.
These findings were validated in mouse experiments. In an induced mouse skin inflammation model, we demonstrated that near-infrared irradiation (energy 60 J/cm2, applied once) reduced the number of inflammatory M1 cells, decreased the inflammatory factor TNF-α, and increased the anti-inflammatory CCL22/TGF-β1. Mice subjected to near-infrared light showed a reduction in redness and other inflammatory symptoms, approaching a state similar to that of non-inflamed skin.
Graphical Abstract
Application and Highlights
1.We discovered the core mechanism through which near-infrared light affects immunity (by epigenetic gene modifications).
2.We exported the basic principle behind near-infrared light's anti-inflammatory effects (by TGF-β1 release)
3.We provided a theoretical basis for the clinical application of near-infrared.
Research Team Members: Wei-Ting Liao, Ph.D., Hsin-Su Yu, M.D., Ph.D.
Representative Department: Department of Biotechnology, Kaohsiung Medical University, Kaohsiung, Taiwan.
Introduction of Research Team:
Professor Wei-Ting Liao's main research focus is on the regulatory roles of macrophages in cancer and inflammatory diseases. Additionally, he reconstructs these regulatory processes using tissue microenvironment models in vitro to evaluate potential prevention and treatment strategies.
Contact Email: wtliao@kmu.edu.tw
Publication: J Invest Dermatol. 2021 Aug; 141(8): 2056-2066.e10
Full-Text Article: https://www.sciencedirect.com/science/article/pii/S0022202X21001573


