以永續又環保方法合成藥物
以永續又環保方法合成藥物
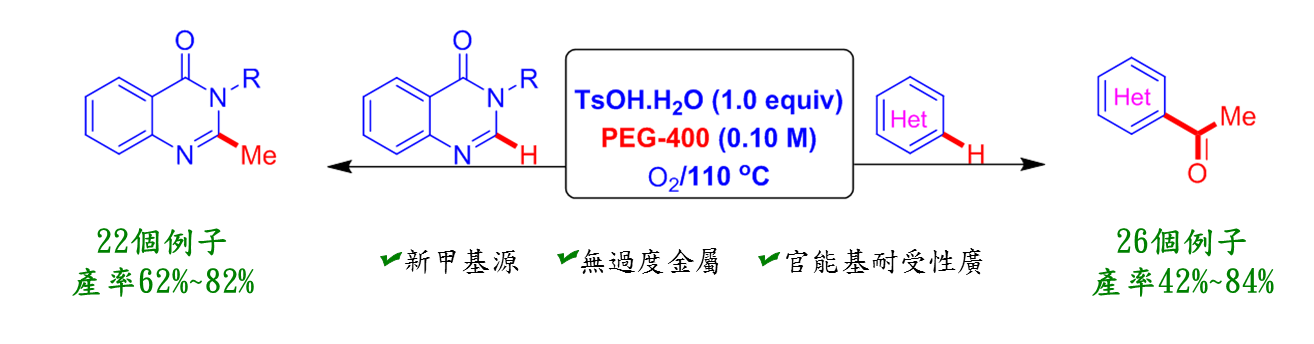
聚乙二醇(PEG)常應用於化妝品與藥物添加劑等用途,是無毒無害的試劑,高醫大研究團隊利用聚乙二醇在對甲苯磺酸與氧氣存在下進行雜芳香族甲基化和乙醯化的新穎且有效的合成途徑。本方法的特點是聚乙二醇不僅做為溶媒,且是這些合成藥物的一個碳或兩個碳來源,許多臨床用藥都具有這類骨架,如: Raltitrexed (抗癌藥物)、Balaglitazone (糖尿病用藥)、Diproquqlone (中樞神經系統抑制劑)等。相信這環保、低價、又有效率的方法能應用到製藥業,提供這類藥物合成的另一種選擇。
圖形摘要:
應用與亮點:
1.聚乙二醇不僅為綠色溶媒,更是低價的化妝品與藥物添加劑。本方法使用聚乙二醇不僅可做為溶媒,又可
提供雜環藥物一個碳或兩個碳來源。
2.這是第一個使用聚乙二醇作為碳源的合成雜環化合物。許多臨床用藥都具有這類骨架,相信這環保且低價
的方法能應用到製藥業,提供藥物合成的另一種選擇。
3.我們也利用有效率一鍋化來合成這類化合物,效果非常好。
【研究團隊】
團隊成員:王志鉦教授和Vishal Suresh Kudale博士
代表單位:高雄醫學大學醫藥暨應用化學系
團隊簡介:高雄醫學大學醫藥暨應用化學系 王志鉦 教授
電話:+ 886-7-3121101分機2275,電子郵件:jjwang@kmu.edu.tw
高雄醫學大學醫藥暨應用化學系 Vishal Suresh Kudale 博士
電話:+886-7-3121101分機2275,電子郵件:vishalkudale90@gmail.com
研究聯繫Email:jjwang@kmu.edu.tw
【論文資訊】
論文出處:Green Chem. 2020 May; 22: 3506-3511.
全文下載:https://doi.org/10.1039/D0GC01183E
Metal-free C–H methylation and acetylation of heteroarenes with PEG-400
Metal-free C–H methylation and acetylation of heteroarenes with PEG-400
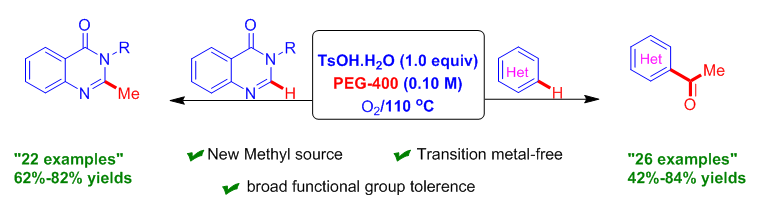
The generation of a methyl carbon source from renewable and cheap sources is challenging. Herein, we describe a novel and an efficient route for methylation and acetylation of aza-heteroarenes using PEG-400 under O2 and TsOH·H2O for the first time by tuning the reaction conditions using a different set of starting materials. The key features of the current protocol are oxidative C–O and C–C bond scission under metal-free conditions with good functional group tolerance, and a broad substrate scope. The potential applicability of the designed methodology was demonstrated for the synthesis of central nervous system (CNS) depressant and anticonvulsant drug molecules by a one-pot strategy.
Graphical Abstract:
Application and Highlights:
Introduction of a methyl group may have a remarkable impact on the biological properties of original molecules. Here we used PEG-400 as the reaction medium. Surprisingly, we observed unusual C-H methylation of quinazolinone and acetylation of other electron-deficient heteroarenes via oxidative C–C and C–O bond cleavage. N-Heteroarenes are important structural moieties observed in pharmaceuticals, natural products, and ligand scaffolds. Quinazolinones are prevalent structural species, and they have immense applications commonly found in medicinal and pharmaceutical chemistry with pharmacological activities, including anti-tumour, anti-inflammatory, anticonvulsant, and antimicrobial activities. A brief literature survey showed that the presence of a methyl group at the C2-position and a substituted aromatic ring at the C3-position are essential functionalities required for CNS depression and anticonvulsant activities of compounds. Advancements using PEG-400 have been achieved because it is environmentally benign in nature; recyclable; is a phase-transfer catalyst; is stable at high temperature; is easily available, has a low cost; and is biodegradable. The PEG-400 used in this work is more eco-friendly than the previously reported methylating reagent. This is the first report to use PEG-400 as a carbon source. Moreover, we demonstrated the potential applicability of the designed protocol for the construction of CNS depressant and anticonvulsant drug compounds from 2-amino-N-arylylbenzamide via sequential Csp2-Csp3 activation in a one-pot manner.
Research Team Members:
Dr. Jeh-Jeng Wang and Vishal Suresh Kudale
Representative Department: Department of Medicinal and Applied Chemistry, Kaohsiung Medical University
Introduction of Research Team:
Dr. Jeh-Jeng Wang, Professor, Department of Medicinal and Applied Chemistry, Kaohsiung Medical University. Tel: +886-7-3121101(Ext: 2275), E-mail: jjwang@kmu.edu.tw
Vishal Suresh Kudale, Doctoral fellow, Department of Medicinal and Applied Chemistry, Kaohsiung Medical University. Tel: +886-7-3121101(Ext: 2275), E-mail: vishalkudale90@gmail.com
Contact Email: jjwang@kmu.edu.tw
Publication: Green Chem. 2020 May 13; 22: 3506-3511.
Full-Text Article: https://doi.org/10.1039/D0GC01183E


