半乳糖凝集素-3在人類免疫缺乏病毒第一型感染所扮演的角色
半乳糖凝集素-3在人類免疫缺乏病毒第一型感染所扮演的角色
半乳糖凝集素(Galectins)是一種會與β-半乳糖苷結合的凝集素,表現於多種細胞,並在多種生理和細胞功能中扮演著一定的角色。此外,半乳糖凝集素也會調控病毒的感染。Galectin-1(Gal-1)、galectin-3(Gal-3)、galectin-8(Gal-8)和galectin-9(Gal-9)被認為是參與病毒感染最主要的半乳糖凝集素,而半乳糖凝集素對於病毒的調控主要是透過在細胞外與病毒糖基化膜蛋白結合、與免疫細胞上的配體或受體相互作用或在細胞內與病毒或細胞內物質作用。半乳糖凝集素對於病毒的調控可能扮演著正向或負向的調控,有一些半乳糖凝集素會根據不同的狀態或位置而具有雙重調控的能力。
Gal-3可調控許多免疫細胞的功能,我們先前發表過,當T細胞受體參與免疫反應時,Gal-3會移至T細胞的免疫突觸並與Alix (ALG-2-interacting protein X)交互作用。Alix是會協調ESCRT(endosomal sorting complex required for transport)進而促進人類免疫缺乏病毒第一型(HIV-1)的病毒顆粒釋放。因此,我們假設 Gal-3會在HIV-1病毒出芽中扮演一定的角色。結果顯示,當Gal-3與HIV-1質體共轉染至Jurkat T細胞會增進HIV-1的病毒出芽,而當Hut78及CD4+ T 細胞利用RNAi抑制Gal-3表現則會使HIV-1的病毒出芽減少。我們也利用免疫螢光顯微鏡發現HIV-1感染後,細胞中Gal-3、Alix 和 Gag 呈現partial colocalization。而免疫共沉澱的結果顯示Gal-3促進Alix與Gag p6的結合,且在Alix knockdown的結果顯示Gal-3是透過Alix而促進HIV-1病毒出芽。HIV-1病毒顆粒從表達Gal-3的細胞中釋放需要以 Alix 依賴的方式獲得Gal-3,而這些蛋白主要存在於病毒體內部。在探討Gal-3與Alix的交互作用發現Gal-3的N端區域會與Alix中富含proline的區域相互作用。綜上所述,結果顯示內源性的Gal-3是透過促進 Alix-Gag p6的結合以促進 HIV-1的病毒出芽。此外,Gal3已被證實會分佈於樹突細胞的膜脂筏 (lipid raft)並促進細胞的遷移。HIV-1已知能透過病毒突觸(Virological Synapse)在T細胞之間傳播,而病毒突觸的完整性取決於脂筏。因此我們也探討Gal-3對於HIV-1在CD4 + T細胞與細胞間傳播(cell-to-cell transmission)的潛在作用。比起在靶細胞(Target cell)中表達Gal-3,在供給細胞(donor cells)中表達Gal-3對於促進HIV-1細胞間轉移更具重要性,且Gal-3能與Gag蛋白一起從HIV-1陽性的供給細胞共同傳播(co-transfer)到HIV-1陰性的靶細胞。我們也發現HIV-1感染會誘導Gal-3與 Gag蛋白一起移動至細胞與細胞的交界並與GM1共定位(co-localization),而GM1是Gal-3促進VS形成的地方,Gal-3也會調控Gag和flotillin-1向細胞膜轉移。最後,Gal-3的減少會降低 CD4+ T細胞膜脂筏中的膽固醇(cholesterol)表現量。上述這些發現證實內源性的Gal-3會調控脂筏的成分並促進HIV-1在CD4+ T細胞之間轉移,也提供了Gal-3調控HIV-1感染的另一條途徑,而這些發現有助於了解Gal-3在HIV-1的感染所扮演的角色以及Gal-3可以成為治療HIV-1感染的標靶。
圖形摘要:
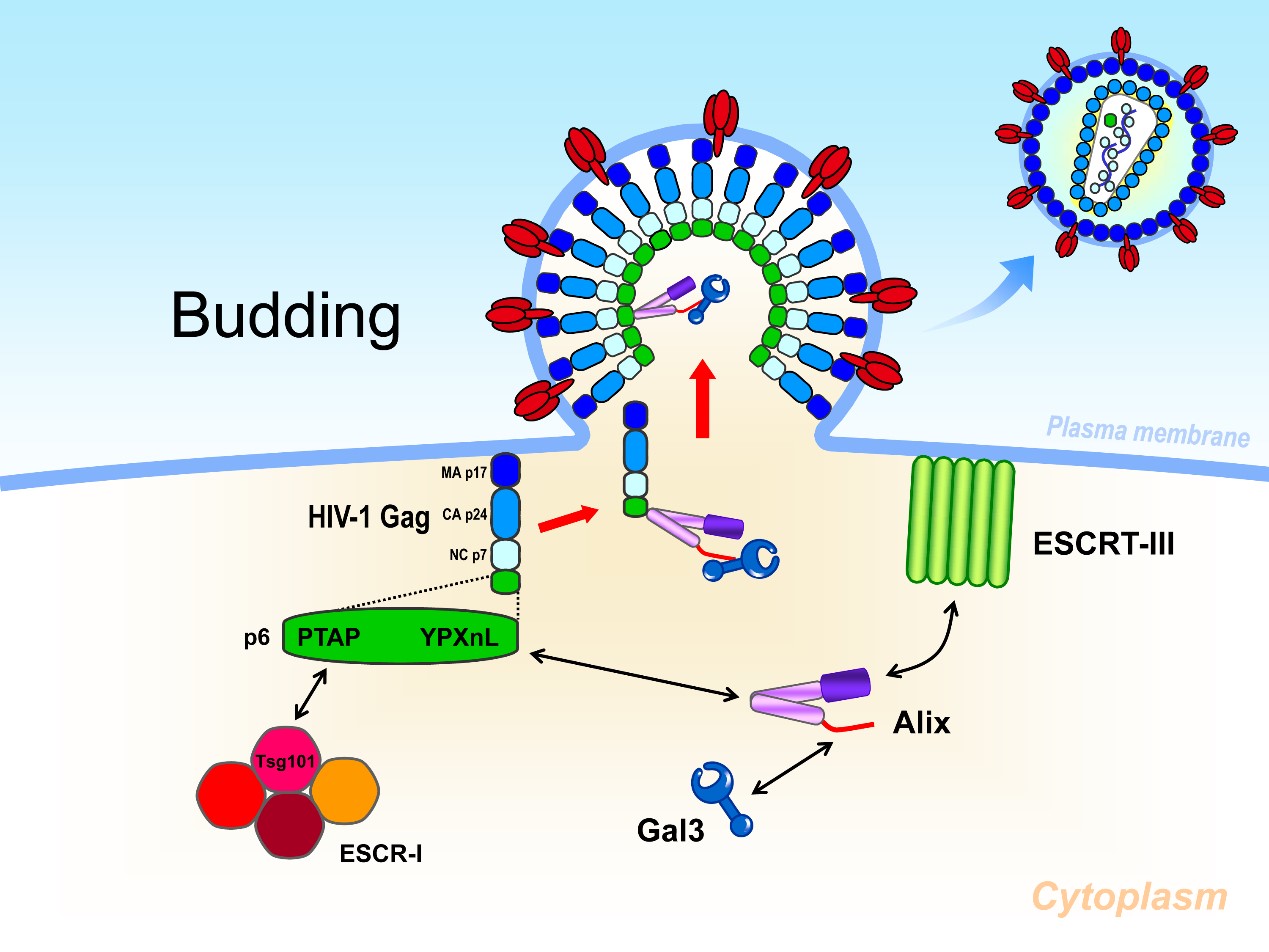
Galectin-3如何調控HIV-1出芽之卡通圖
Galectin-3的N端區域可以和Alix的proline-rich 區域結合並穩定Alix-Gag的交互作用進而促進HIV-1病毒出芽
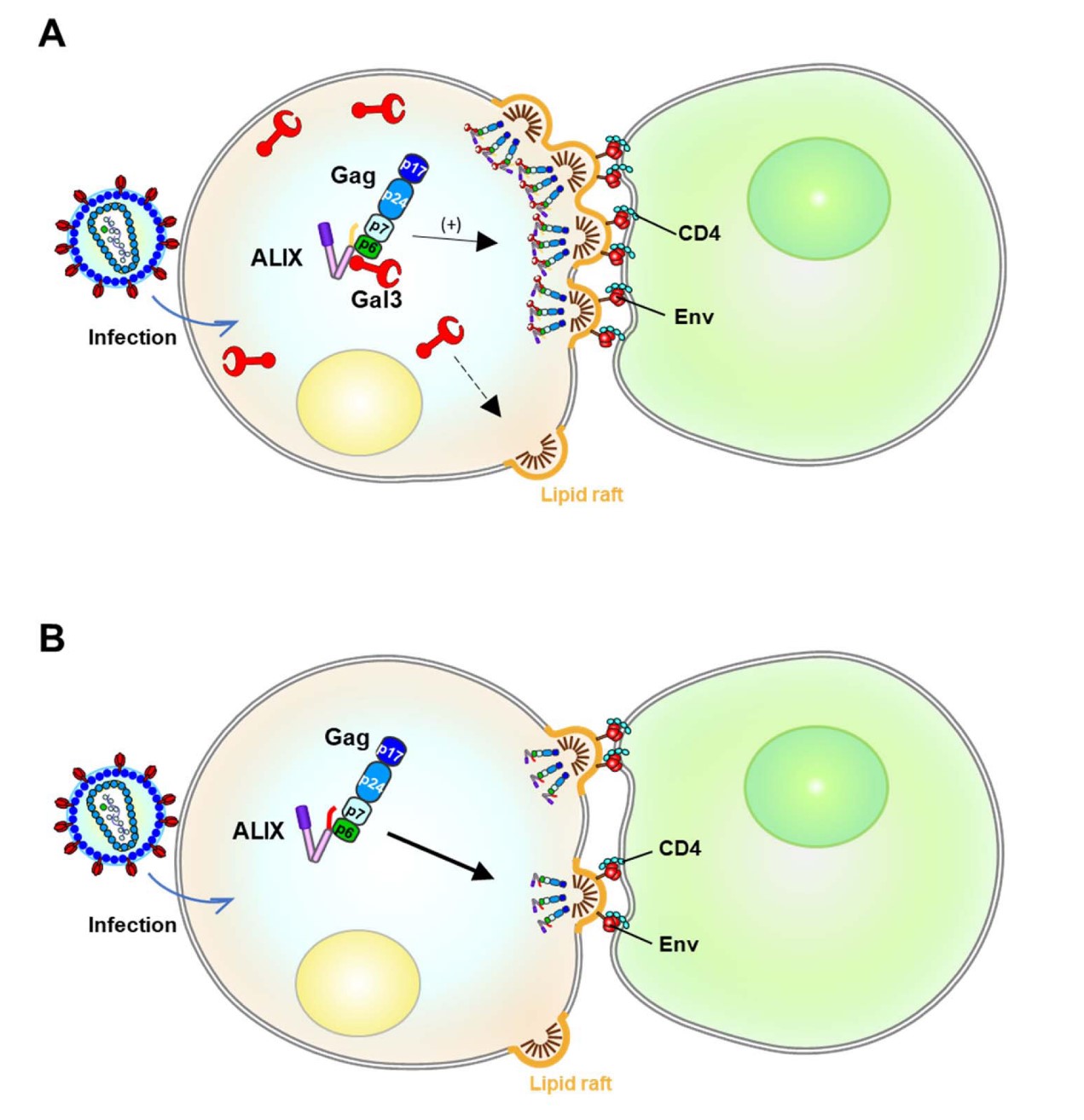
Galectin-3 增強脂筏表達和極化促進 HIV-1 的細胞與細胞接觸傳播
(A) 具有 Gal3 表達的 HIV-1+ 供體細胞通過細胞間接觸感染靶細胞。 (B) 沒有 Gal3 表達的 HIV-1+ 供體細胞通過細胞間接觸感染靶細胞。
應用與亮點:
1. Galectin-3促進Alix和Gag p6的結合進而促進HIV-1病毒出芽。
2. Galectin-3促進HIV-1在CD4 T細胞中細胞與細胞間傳遞。
【研究團隊】
團隊成員: 楊子萱, 林芷妍, 王文宏, Wanchai Assavalapsakul, Arunee Thitithanyanont, 盧柏樑, 陳彥旭, 林俊宏, 陳煥源, 劉扶東, 王聖帆
代表單位:高雄醫學大學 醫學檢驗生物技術學系
團隊簡介:此研究之團隊包括: (1)高雄醫學大學醫學檢驗生物技術學系、(2)高雄醫學大學熱帶醫學暨傳染病研究中心、(3)高雄醫學大學附設醫院感染內科、(4)泰國朱拉隆功大學微生物學系、(5)泰國瑪希敦大學微生物學系、(6)中央研究院生物醫學研究所與(7)中央研究院生物化學化研究所。
研究聯繫Email:wasf1234@kmu.edu.tw
【論文資訊】
論文出處:
Glycobiology, 2022 June; cwac040
Glycobiology, 2014 July; Volume 24, Issue 11, Pages 1022–1035
Journal of Microbiology, Immunology and Infection, 2020 December, Volume 53, Issue 6, Pages 925-935
全文下載:
https://doi.org/10.1093/glycob/cwac040
https://doi.org/10.1093/glycob/cwu064
https://doi.org/10.1016/j.jmii.2019.09.005
The Roles of Galectin-3 In HIV-1 Infection
The Roles of Galectin-3 In HIV-1 Infection
Galectins are β-galactoside-binding lectins expressed in numerous cells and play multiple roles in various physiological and cellular functions. In addition, galectins play multiple roles in regulation of virus infections. Galectin-1 (Gal-1), galectin-3 (Gal-3), galectin-8 (Gal-8), and galectin-9 (Gal-9) were found as the most predominant galectins reported to participate in virus infection. The regulatory function of galectins occurs by extracellularly binding to viral glycosylated envelope proteins, interacting with ligands or receptors on immune cells, or acting intracellularly with viral or cellular components in the cytoplasm. Several galectins express either positive or negative regulatory role, while some had dual regulatory capabilities on virus propagation based on the conditions and their localization.
Gal-3 has been reported to regulate the functions of a number of immune cell types. We previously reported that Gal-3 is translocated to immunological synapses in T cells upon T-cell receptor engagement, where it associates with ALG-2-interacting protein X (Alix). Alix is known to coordinate with the endosomal sorting complex required for transport (ESCRT) to promote human immunodeficiency virus (HIV)-1 virion release. Therefore, we hypothesized that Gal-3 plays a role in HIV-1 viral budding. The results showed that co-transfection of Jurkat T cell line with Gal-3 and HIV-1 plasmids increased HIV-1 budding, and suppression of Gal-3 expression by RNAi in Hut78 and primary CD4+ T cells led to reduced HIV-1 budding. We also used immunofluorescence microscopy to observe the partial colocalization of Gal-3, Alix and Gag in HIV-1-infected cells. The results from co-immunoprecipitation experiments indicate that Gal-3 expression promotes Alix-Gag p6 association, whereas the results of Alix knockdown suggest that Gal-3 promotes HIV-1 budding through Alix. HIV-1 particles released from Gal-3-expressing cells acquire the Gal-3 protein in an Alix-dependent manner, with proteins primarily residing inside the virions. We also found that the Gal-3 N-terminal domain interacts with the proline-rich region of Alix. Collectively, these results suggest that endogenous Gal-3 facilitates HIV-1 budding by promoting the Alix-Gag p6 association. Besides, Gal3 has been shown to localize in membrane lipid rafts in dendritic cells and positively regulate cell migration. HIV-1 spreads between T cells by forming supramolecular structures (virological synapses [VSs]), whose integrity depends on lipid rafts. Therefore, we were going to address the potential role of Gal-3 in cell-to-cell transmission of HIV-1 in CD4 T cells. Gal-3 expressed in donor cells was more important for facilitating HIV-1 cell-to-cell transfer than Gal-3 expressed in target cells. Gal-3 was found to be co-transferred with Gag from HIV-1-positive donor to HIV-1-negative target T cells. HIV-1 infection induced translocation of Gal-3 together with Gag to the cell–cell interfaces and colocalize with GM1, where Gal-3 facilitated VS formation. Gal-3 regulated the coordinated transfer of Gag and flotillin-1 into plasma membrane fractions. Finally, depletion of Gal-3 reduced the cholesterol levels in membrane lipid rafts in CD4 T cells. These findings provide evidence that endogenous Gal-3 stimulates lipid raft components and facilitates intercellular HIV-1 transfer among CD4 T cells, offering another pathway by which Gal-3 regulates HIV-1 infection. These findings may inform the treatment of HIV-1 infection based on targeting Gal-3 to modulate lipid rafts.
Graphical Abstract:

Scheme of how galectin-3 dependent on Alix to promote HIV-1 budding
Galectin-3 N-terminal domain interacts with the proline-rich region of Alix. This interaction stabilizes Alix-Gag interaction then benefits HIV-1 budding.

Galectin-3 enhances lipid raft expression and polarization and further
promote HIV-1 cell-to-cell transmission. (A) The HIV-1+ donor cells with Gal3 expression infected to the target cells via cell-cell contact. (B) The HIV-1+ donor cells without Gal3 expression infected to the target cells via cell-cell contact.
Application and Highlights:
1. Galectin-3 promotes Alix and Gag p6 association and facilitated HIV-1 budding.
2. Galectin-3 facilitates HIV-1 cell-to-cell transmission in CD4 T cells.
Research Team Members: Zih-Syuan Yang, Chih-Yen Lin, Wen-Hung Wang, Wanchai Assavalapsakul, Arunee Thitithanyanont, Po-Liang Lu, Yen-Hsu Chen, Chun-Hung Lin, Huan-Yuan Chen, Fu-Tong Liu and Sheng-Fan Wang
Representative Department: Department of Medical Laboratory Science and Biotechnology, Kaohsiung Medical University.
Introduction of Research Team: The research team includes (1) Department of Medical Laboratory Biotechnology, Kaohsiung Medical University, (2) Center for Tropical Medicine and Infectious Diseases, Kaohsiung Medical University, (3) Division of Infectious Disease, Department of Internal Medicine, Kaohsiung Medical University Hospital, (4) Department of Microbiology, Chulalongkorn University, Thailand, (5) Department of Microbiology, Mahidol University, Thailand, (6) Institute of Biomedical sciences and (7) Institute of Biological Chemistry, Academia Sinica.
Contact Email: wasf1234@kmu.edu.tw
Publication:
Glycobiology, 2022 June; cwac040
Glycobiology, 2014 July; Volume 24, Issue 11, Pages 1022–1035
Journal of Microbiology, Immunology and Infection, 2020 December, Volume 53, Issue 6, Pages 925-935
Full-Text Article:
https://doi.org/10.1093/glycob/cwac040
https://doi.org/10.1093/glycob/cwu064
https://doi.org/10.1016/j.jmii.2019.09.005


