利用單細胞RNA定序表徵化EGFR突變肺癌的肋膜微環境與癌症轉變
利用單細胞RNA定序表徵化EGFR突變肺癌的肋膜微環境與癌症轉變
肺癌是一種影響全球的複雜癌症,當肺癌細胞轉移至肋膜腔時,導致惡性肋膜積液(malignant pleural effusion, MPE),病人會出現胸痛、呼吸急促和健康狀況變差等症狀。此外,惡性肋膜積液的產生,將導致更差的臨床預後和生活品質相關。儘管有越來越多的研究致力於探索其細胞機制,對於肺癌惡性肋膜積液其了解仍不完整。
由於當前生物技術的迅速發展,單細胞RNA定序(scRNA-seq)已成為探索單細胞基因表達的重要工具。此外,scRNA-seq是一種強大且高解析的工具,能夠發現特定細胞或是細胞之間的相互作用。在此研究中,我們收集了來自四名患有表皮生長因子受體(epithelial growth factor receptor, EGFR)突變的肺癌患者和四名心衰竭患者(作為對照組)的肋膜積液檢體,並收集了三名患者的非腫瘤相鄰肺組織和腫瘤組織,來探討轉移過程中的變化。此外,生物資訊分析用於計算細胞分化、相關訊息路徑和不同的配體-受體相互作用。再者,通過酶聯免疫吸附法(ELISA)或多細胞因子磁珠試驗法檢測肋膜積液中的可溶性因子,有助於比較樣本之間的蛋白質表現。最後,西方墨點法和免疫組織化學染色(immunohistochemical staining, IHC staining)用於驗證scRNA-seq的分析結果。
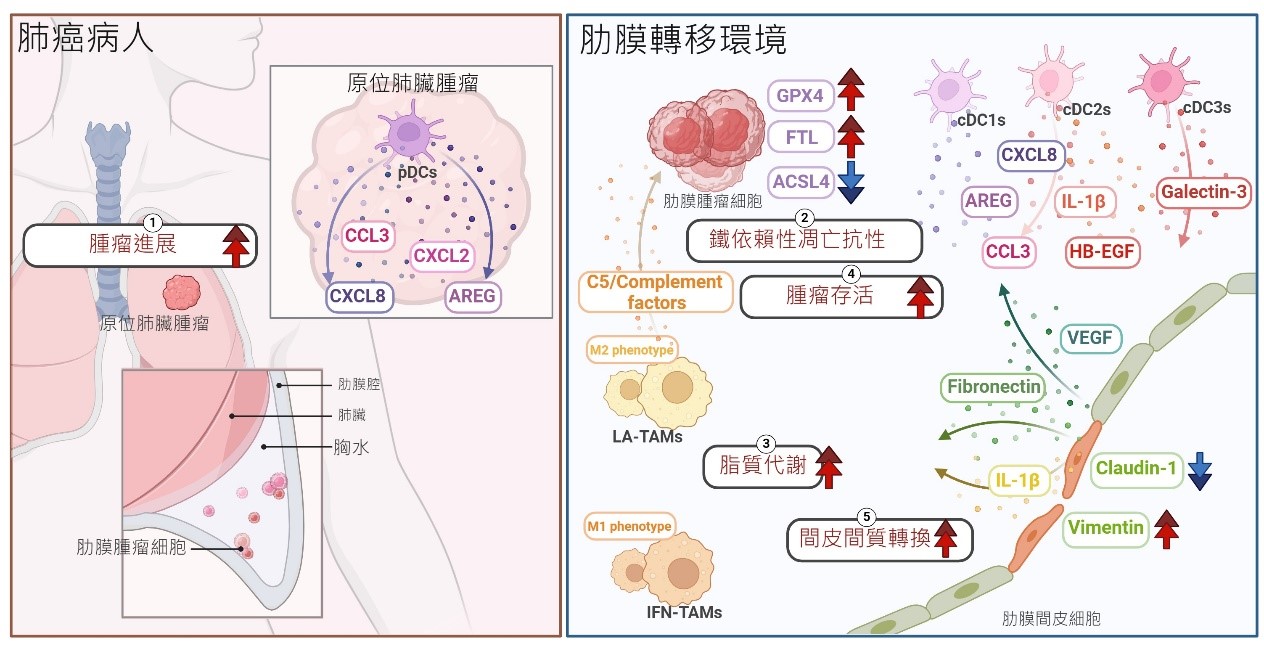
在結果中,間皮細胞透過增加VIM、FN1和VEGFA的表現,證明間皮-間質轉化(MesoMT),可以協助癌細胞肋膜轉移。與原發性肺癌細胞相比,肺癌肋膜轉移癌細胞藉由上調GPX4,表現出鐵依賴性細胞凋亡抗性,從而防止在轉移過程中的細胞死亡。另一方面,骨髓系免疫細胞被分為十八個獨特的細胞群,展示出不同的吞噬和抗原呈現能力。此外,脂質相關的腫瘤相關巨噬細胞(LA-TAMs)不僅呈現M2表型,而且在路徑分析中與膽固醇代謝和補體級聯相關,代表LA-TAMs可能在MPE中提供有利的腫瘤微環境。有趣的是,EGFR突變患者中的補體因子C5,其高表現與更差的存活率相關。在軌跡分析中,ZNF331在漿狀樹突細胞(pDCs)中的表達較高,可能在原發性肺癌患者中調節抗腫瘤免疫功能。最後,在細胞間相互作用分析中,間皮細胞透過TNC和ICAM1與MPE中的癌細胞和免疫細胞進行交互作用。
總結來說,這項研究在單細胞層次上提供了肺癌胸膜轉移的藍圖,並為未來藥物開發提供了許多可能的治療標的。
圖形摘要
應用與亮點:
1.間皮細胞表現間皮-間質轉化,有助於癌症轉移。
2.肺癌細胞在轉移到肋膜過程中表現ferroptosis抗性。
3.LA-TAMs呈現M2表型,與膽固醇代謝和補體級聯相關。
4.C5的高表達與EGFR突變患者的存活率相關。
5.這項研究提供了許多可能的肺癌患者治療標的。
【研究團隊】
團隊成員:
吳宇瑗、許雅玲、黃永琪、蘇月秋、吳寬澧、張昭元、翁采彤、賴佳辰、沈子晏、李岱晃、洪仁宇、蔡英明
代表單位:高雄醫學大學新藥開發暨價創研究中心
研究團隊:
許雅玲教授及其實驗室團隊不僅致力於多種癌症的研究,如肺癌、乳癌,特別聚焦研究在腫瘤微環境和癌症轉變上,除此之外,也研究氣喘的病理機制,並尋找疾病可能的治療標的。
研究聯繫:yingming@kmu.edu.tw yainghsu@kmu.edu.tw
Publication: Theranostics. 2023 Aug; 13 (13): 4412-4429.
Full-Text Article:
1.https://www.thno.org/v13p4412.htm
2.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10465223/
3.https://doi.org/10.7150/thno.85084
Characterization of the pleural microenvironment niche and cancer transition using single-cell RNA sequencing in EGFR-mutated lung cancer
Characterization of the pleural microenvironment niche and cancer transition using single-cell RNA sequencing in EGFR-mutated lung cancer
Lung cancer is a complex form of cancer that affects people worldwide. When lung cancer cells metastasize into the pleural cavity, it can result in malignant pleural effusion (MPE), leading to symptoms such as chest pain, shortness of breath, and a decline in physiological condition. Moreover, MPE is associated with a poorer clinical prognosis and life quality. Despite an increasing number of studies dedicated to exploring the cellular mechanisms of MPE, our understanding remains incomplete.
Due to the swift advancement of biotechnologies nowadays, single-cell RNA sequencing (scRNA-seq) has become instrumental in exploring gene expression at the individual cell level. Moreover, scRNA-seq serves as a potent and high-resolution tool capable of identifying specific cell types and elucidating distinct cell-cell interactions. In our research, we obtained pleural effusion samples from four lung cancer patients with epidermal growth factor receptor (EGFR) mutations and four patients with heart failure, who were used as the control group. Additionally, adjacent non-tumor lung tissues and paired tumor tissue from three patients were collected, enabling us to compare the underlying changes within cancer transition. Furthermore, bioinformatic analyses, such as trajectory analysis, pathway analysis, and cell-cell interaction analysis, were used to calculate cell differentiation, associated signaling pathways, and distinct ligand-receptor interactions, respectively. In addition, specific soluble factors in pleural effusion were detected by enzyme-linked immunosorbent assay (ELISA) or multiplex cytokine bead assay, aiding in the comparison of protein levels among samples. Ultimately, western blot and immunohistochemistry (IHC) staining were applied to validate the results in scRNA-seq data.
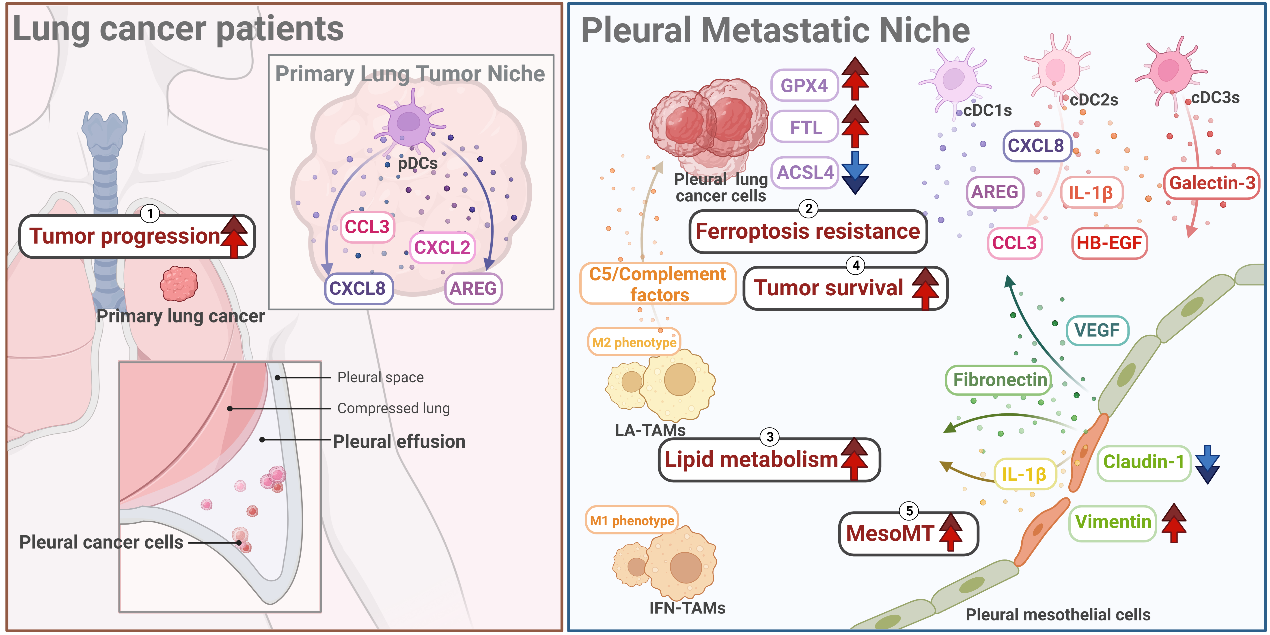
In the results, mesothelial cells, a unique cell type constituting the pleura by lining the surfaces of the lung, exhibited mesothelial-mesenchymal transition (MesoMT) by increasing the expression of VIM, FN1, and VEGFA, aiding in the metastasis of cancer cells. Additionally, changes of ferroptosis, an iron-dependent cell death caused by oxidative stress, was observed. Pleural cancer cells demonstrated ferroptosis resistance by up-regulating GPX4, a ferroptosis-suppressed gene, preventing cell death during metastasis compared to primary lung cancer cells. On the other hand, myeloid-lineage immune cells, such as dendritic cells, monocytes, and macrophages, were divided into eighteen unique clusters, demonstrating distinct abilities of phagocytosis and antigen-presenting capability. Moreover, compared with interferon-primed tumor-associated macrophages (IFN-TAMs), lipid-associated tumor-associated macrophages (LA-TAMs) not only displayed M2 phenotypes but also correlated with cholesterol metabolism and complement cascades in pathway analysis. This suggests that LA-TAMs could provide a pro-tumor microenvironment in MPE. Additionally, complement factors showed higher levels universally in multiple soluble factor analyses. Interestingly, a high level of complement factor C5 was associated with worse survival in EGFR-mutated patients. Furthermore, each type of dendritic cells expressed higher levels of different gene expressions and soluble proteins in single-cell data and multiplex soluble factor assays, respectively. In the trajectory analysis, ZNF331 expressed higher in the plasmacytoid dendritic cells (pDCs), possibly regulating anti-tumor immune function in primary lung cancer patients. Finally, in the overall cell-cell interaction analysis, mesothelial cells communicated with cancer cells and immune cells in MPE through TNC and ICAM1.
In conclusion, this study provides a blueprint containing unique cellular and molecular characteristics in pleural metastasis and offers numerous therapeutic targets for future drug development.
Graphical Abstract
Application and Highlights:
1.Mesothelial cells exhibited Mesothelial-mesenchymal transition helping cancer metastasis.
2.Pleural cancer cells demonstrated ferroptosis resistance during cancer transition.
3.LA-TAMs had a M2 phenotype and correlated with cholesterol metabolism and complement cascades.
4.Higher expression of C5 associated with poor survival in EGFR-mutated patients.
5.This study provided lots of possible therapeutic targets for lung cancer patients.
Research Team Members:
Yu-Yuan Wu, Ya-Ling Hsu, Yung-Chi Huang, Yue-Chiu Su, Kuan-Li Wu, Chao-Yuan Chang, Chai-Tung Ong, Jia-Chen Lai, Tzu-Yen Shen, Tai-Huang Lee, Jen-Yu Hung, Ying-Ming Tasi
Representative Department:
Drug Development and Value Creation Research Center, Kaohsiung Medical University
Introduction of Research Team:
Professor Ya-Ling Hsu and her laboratory team are not only devoted to researching various cancers, including lung cancer and breast cancer, with a particular focus on tumor microenvironment and cancer transition, but also make efforts to study the pathogenesis of asthma and identify potential therapeutic targets.
Contact Email: yingming@kmu.edu.tw ; yainghsu@kmu.edu.tw
Publication: Theranostics. 2023 Aug; 13 (13): 4412-4429.
Full-Text Article:
1.https://www.thno.org/v13p4412.htm
2.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10465223/
3.https://doi.org/10.7150/thno.85084


