研究脂肪幹細胞載體於GelMA/HAMA/PEGDA光固化生物列印墨水中向平滑肌細胞表型的分化能力
研究脂肪幹細胞載體於GelMA/HAMA/PEGDA光固化生物列印墨水中向平滑肌細胞表型的分化能力
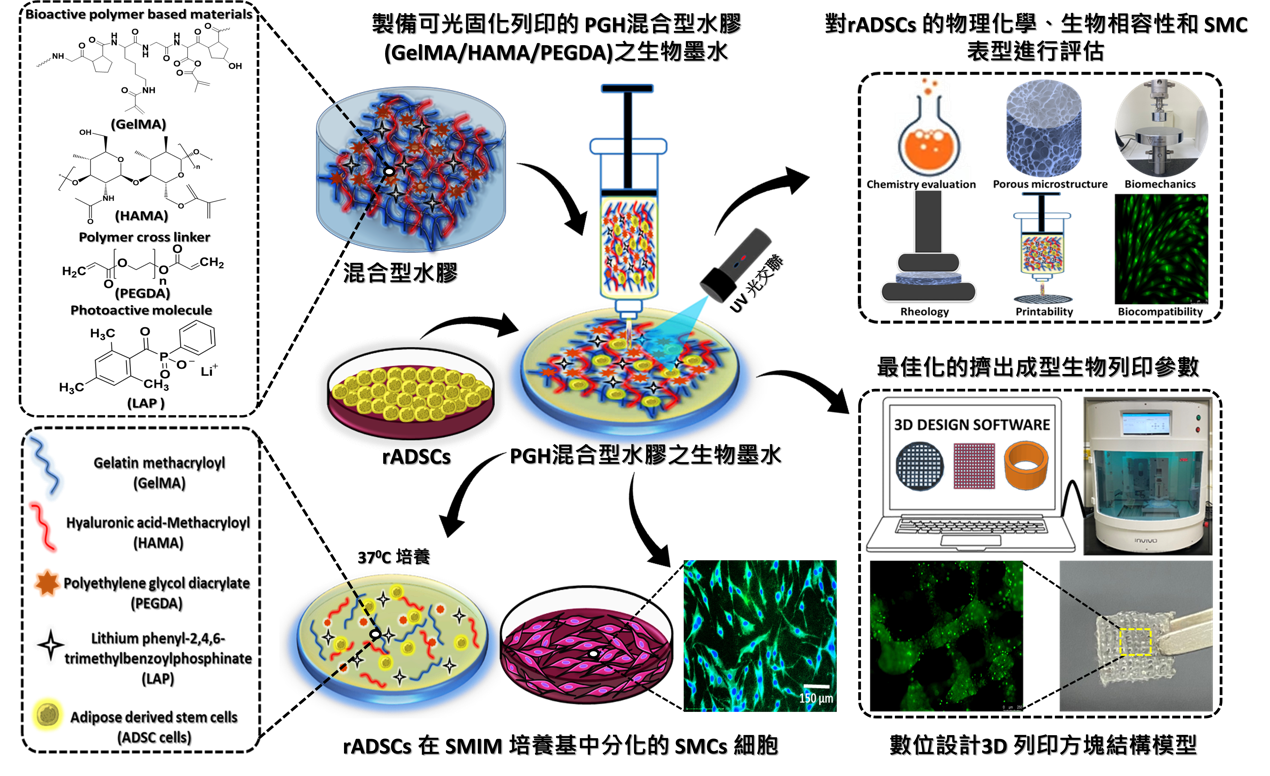
本研究開發了一種基於聚合物的光交聯三維列印支架,該支架由明膠甲基丙烯醯(gelatin methacryloyl, G)、透明質酸甲基丙烯醯(hyaluronic acid methacryloyl, H)和兩種分子量的聚乙二醇二丙烯酸酯(polyethylene glycol diacrylate, P),以及不同濃度的P組成。此支架可促進來自紐西蘭白兔脂肪幹細胞(rADSCs)存活、增殖並分化成平滑肌細胞(smooth muscle cells, SMCs)。研究中對PGH生物墨水進行了化學改性及其理化性質的評估,並透過MTT和CCK-8粒線體活性測試以及活/死細胞(Live/Dead)染色法評估細胞活性。此外,使用TRAP (tartrate-resistant acid phosphatase)染色法探討了分化後SMCs的形態和細胞核數量,並通過定量RT-PCR分析了兩種SMCs特異性基因標記(α-SMA和SM-MHC)的表達,以評估基因表達水準。並利用免疫螢光染色(Immunocytofluorescence, ICC)和西方墨點法(western blotting)技術研究了SMCs特異性蛋白(α-SMA和SM-MHC)的分化能力。最後,以擠出成型式的生物列印技術成功驗證含脂肪幹細胞(rADSC)於PGH7 (GelMA/HAMA=2/1) + PEGDA-8000 Da, 3% w/v) 混合生物墨水的3D結構模型(圓盤、方塊和管狀),並驗證其細胞存活能力佳。本研究成功開發在具有良好生物相容性的PGH7混合生物墨水中,SMCs的整體活性和表型得以保持,顯示出其在平滑肌組織再生和相關器官修復領域中具有相當應用潛力。
圖形摘要
應用與亮點:
1.已分化的SMC在PGH7混合水凝膠支架中保持了紡錘體形態,並保留了SM表型標記,該支架具有出色的控制釋放性能和最佳的機械性能。
2.PGH7支架兼顧了孔徑大小、支架硬度和最佳機械性能,可顯著提高SMC的存活率和擴散能力。
3.這項研究有效地解決了SMCs研究中常見的倫理限制和缺乏可重複性的問題。
4.我們的研究結果將對SMCs在SM組織(氣管、血管等)相關再生中的成功再生潛力產生廣泛影響。
5.此外,將來還可以將它們植入動物SM組織相關缺陷模型中。
研究團隊
團隊成員:Pavanchandh Atturu, Sunaina Mudigonda, 王昭仁, 吳順成, 陳振偉, Mary Fornica Francis Forgia, Hans-Uwe Dahms, 王志光
代表單位:再生醫學與細胞治療研究中心
研究聯繫Email:ckwang@kmu.edu.tw
論文出處:International Journal of Biological Macromolecules Volume 265, Part 2, April 2024, 130710
全文下載:https://doi.org/10.1016/j.ijbiomac.2024.130710
Adipose-derived stem cells loaded photocurable and bioprintable bioinks composed of GelMA, HAMA and PEGDA crosslinker to differentiate into smooth muscle phenotype
Adipose-derived stem cells loaded photocurable and bioprintable bioinks composed of GelMA, HAMA and PEGDA crosslinker to differentiate into smooth muscle phenotype
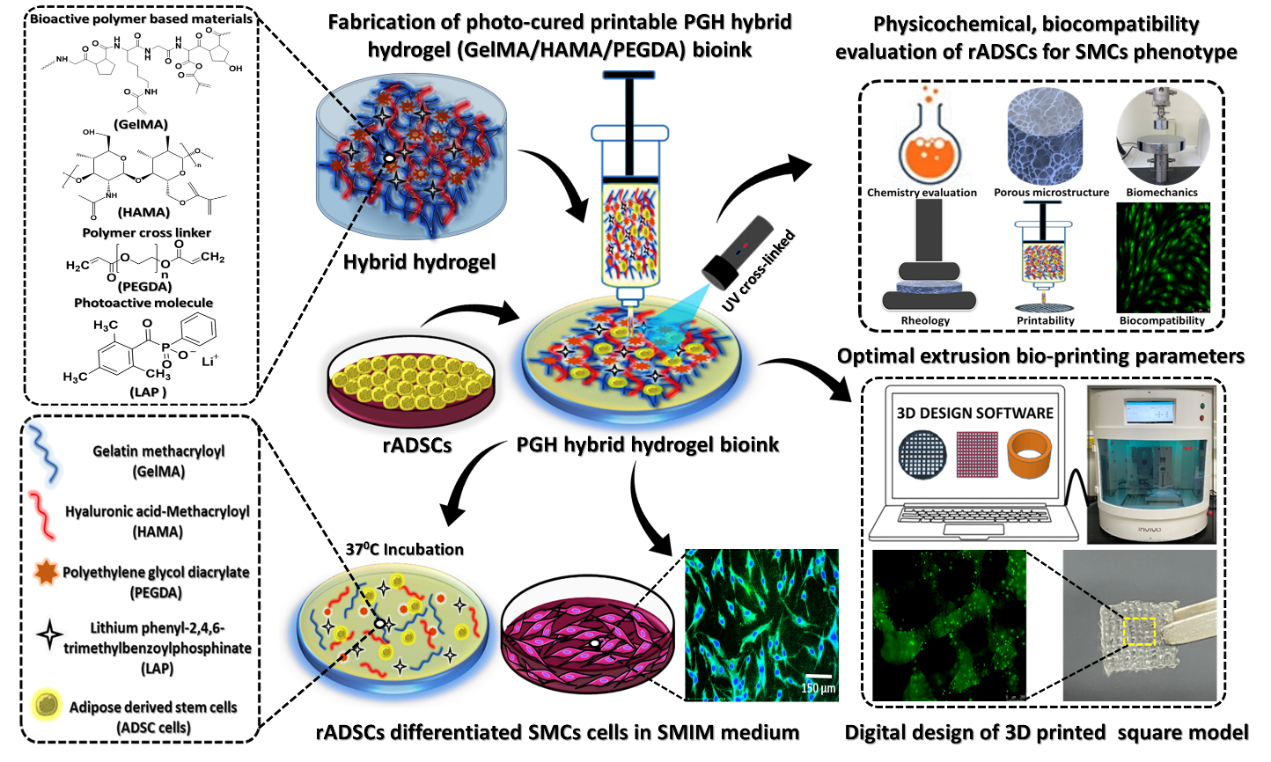
Developing a polymer-based photocrosslinked 3D printable scaffolds comprised of gelatin methacryloyl (G) and hyaluronic acid methacryloyl (H) incorporated with two molecular weights of polyethylene glycol diacrylate (P) of various concentrations that enables rabbit adipose-derived stem cells (rADSCs) to survive, grow, and differentiate into smooth muscle cells (SMCs). Then, the chemical modification and physicochemical properties of the PGH bioinks were evaluated. The cell viability was assessed via MTT, CCK-8 assay and visualized employing Live/Dead assay. In addition, the morphology and nucleus count of differentiated SMCs were investigated by adopting TRAP (tartrate-resistant acid phosphatase) staining, and quantitative RT-PCR analysis was applied to detect gene expression using two different SMC-specific gene markers α-SMA and SM-MHC. The SMC-specific protein markers namely α-SMA and SM-MHC were applied to investigate SMC differentiation ability by implementing Immunocytofluorescence staining (ICC) and western blotting. Moreover, the disk, square, and tubular cellular models of PGH7 (GelMA/HAMA=2/1) + PEGDA-8000 Da, 3% w/v) hybrid bioink were printed using an extrusion bioprinting and cell viability of rADSCs was also analysed within 3D printed square construct practising Live/Dead assay. The results elicited the overall viability of SMCs, conserving its phenotype in biocompatible PGH7 hybrid bioink revealing its great potential to regenerate SMCs associated organs repair.
Graphical Abstract
Application and Highlights:
1.Differentiated SMCs maintained their spindle morphology and preserved their SM phenotypic markers in the PGH7 hybrid hydrogel scaffold, which exhibited excellent controlled release performance and optimal mechanical properties.
2.With a balance of pore size, scaffold stiffness, and optimal mechanical properties, the PGH7 scaffold significantly maintained enhanced viability and spreading of SMCs over time.
3.This study effectively addressed limitations associated with ethical constraints and the lack of reproducibility commonly found in SMCs research.
4.Our findings will have broad implications for the successful regenerative potential of SMCs in SM tissue (tracheal, vascular, and beyond) associated regeneration.
5.Furthermore, they can be implanted in animal SM tissue-associated defect models in the future.
Research Team Members: Pavanchandh Atturu, Sunaina Mudigonda, Chau-Zen Wang, Shun-Cheng Wu, Jhen-Wei Chen, Mary Fornica Francis Forgia, Hans-Uwe Dahms, Chih-Kuang Wang
Representative Department: Regenerative Medicine and Cell Therapy Research Center (RCC)
Contact Email: ckwang@kmu.edu.tw
Publication:International Journal of Biological Macromolecules Volume 265, Part 2, April 2024, 130710
Full-Text Article:https://doi.org/10.1016/j.ijbiomac.2024.130710


