癌細胞轉移的誘發複合體(ISX-BRD4)參與腫瘤細胞微環境之建構並促使腫瘤細胞進行轉移及癌症惡化
癌細胞轉移的誘發複合體(ISX-BRD4)參與腫瘤細胞微環境之建構並促使腫瘤細胞進行轉移及癌症惡化
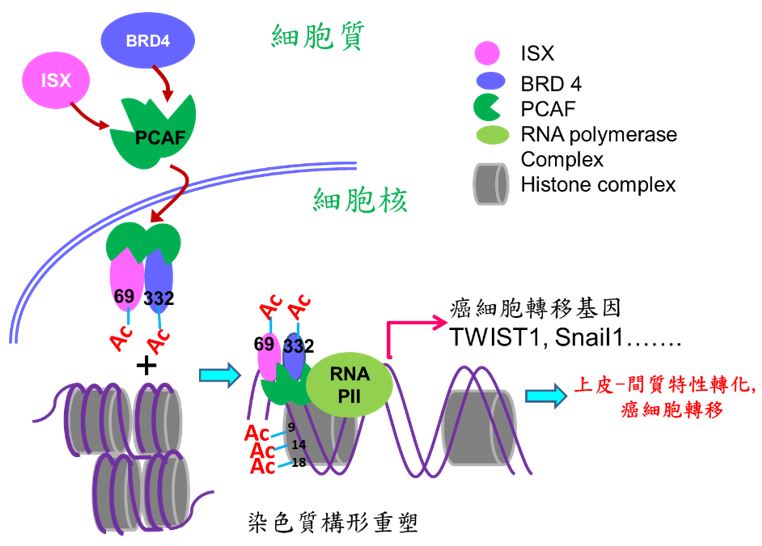
在大多數腫瘤所導致的預後不良及相關死亡中,首要原因(90%)是癌症細胞的轉移所導致。儘管有關於癌症轉移的相關研究日益增多,造成腫瘤轉移的作用機制尚不明確。癌症細胞的轉移是一個複雜過程,而癌細胞的上皮-間質特性轉化(EMT)被認為是最初也是至為重要的步驟。儘管許多信息傳遞分子及路徑(例如,PI3K,Snail,HIF1α,和 SIP1)和轉錄因子(TWIST1/ 2, SNAIL1/ 2,ZEB1/ 2,和 FOXC2)被認為是調節及誘導 EMT 發生的主要因子,詳細的調控機制仍是未知。表觀基因調控(Epigenetic regulation)是癌症進展過程中的重要調節過程,特別是蛋白質乙醯化的機制,但是,其潛在機制亦尚未充分了解。
本校醫學研究所許世賢教授利用免疫沉澱法、質譜分析(LC-Mass/Mass)及鄰位連接技術(Proximity Ligation Assay; PLA)等分子生物學技術發現一個新的致癌複合體(ISX-BRD4),此複合體可誘發及參與腫瘤細胞微環境之建構,使腫瘤細胞進行轉移及惡化。箱型基因Intestine -specific homeobox (ISX) 是一個許世賢教授實驗室發現及證實的一個致癌小腸專一性表現之箱型基因,實驗室多年的研究發表中證實此轉錄因子可經由發炎因子(IL-6)所誘發並且高量表現在肝癌細胞及病人組織中。在細胞核中,經由直接結合下游細胞週期因子(cyclin D1)之啟動子,ISX 的表現可調控癌細胞的增生並與病人之生存時間、腫瘤大小及腫瘤期別有著緊密的調控關係。近期研究發現表觀乙醯轉移酶P300 / CBP相關因子(PCAF)可經由調控ISX(K69)與BRD4(K332)複合物之乙醯化,促使ISX-BRD4複合體進入細胞核,與EMT調控基因啟動子結合並進一步乙醯化組織蛋白3(Histone H3; K9, 14, 18),促使染色質雙股結構鬆開,開始EMT相關調控基因轉錄促進癌症轉移。過度表現ISX會增強EMT標記物的表現,包括EMT 調控基因TWIST1,Snail1, Slug, ZEB1和VEGF,以及隨之而來的癌症轉移,但會抑制E-鈣黏著蛋白(E-cadherin)的表現。 在肺癌中,PCAF–ISX–BRD4複合體過度表達與臨床轉移特徵和不良預後呈現高度相關性。這些結果證實,PCAF–ISX–BRD4複合體表現確實加強了EMT信號傳導,並對腫瘤的發生和轉移發揮了調節作用。
論文全文:https://pubmed.ncbi.nlm.nih.gov/31908141/
圖說:根據實驗數據和結構建模的實驗發現,BRD4的BD2域上的酪氨酸(Tyr390)和天冬醯胺(Asn433)殘基是BRD4識別並與ISX的乙酰化賴氨酸(Lys69)結合併促進ISX–BRD4複合物形成所需的關鍵殘基。
本篇為高雄醫學大學2020年月傑出論文2月份得獎文章,代表作者為醫學院醫學研究所許世賢教授。
本校主要研究者之簡介:
許世賢 教授, 高雄醫學大學 醫學研究所
研究聯繫Email:
許世賢教授 (jackhsu@kmu.edu.tw), TEL:07-3121101#2136
期刊出處: EMBO Rep. 2020 Feb 5;21(2):e48795.
研究全文下載:
https://pubmed.ncbi.nlm.nih.gov/31908141/
PCAF-mediated acetylation of ISX recruits BRD4 to promote epithelial-mesemnchymal transition
PCAF-mediated acetylation of ISX recruits BRD4 to promote epithelial-mesemnchymal transition
Cancer directly affects at least one-third of the human population. Despite this extensive research prevalence, the genetic determinants of cancer risk remain largely unknown. Metastasis, a complex, multistep morphogenetic process, refers to the dissemination from a primary tumor mass to distal tissues, with epithelial–mesenchymal transition (EMT) believed to be the initial and vital step. Despite several signaling (e.g., PI3K, snail, HIF1α, and SIP1) and transcriptional (TWIST1/2, SNAIL1/2, ZEB1/2, and FOXC2) factors having been identified as major regulators of EMT, a detailed regulatory mechanism for oncogene-induced EMT has yet to be established. Epigenetic regulation is an important process during cancer progression, however, the underlying mechanisms, particularly those involving protein acetylation, remain to be fully understood. Recently, Prof. Hsu (GIM, KMU) found intestine-specific homeobox (ISX), a newly identified pair-family homeobox transcription factor, is a pro-inflammatory cytokine (IL-6) induced homeobox gene and high expression in hepatoma cells and HCC patients. Through directly regulates downstream cell cycle regulators (cyclin D1 and E2F1), ISX shows high correlation to patient survival time, tumor size, tumor number and progression stage, and accelerated cell proliferation and tumorigenic activity in hepatoma cells which highlights ISX is an important regulator in hepatoma progression with significant potential as a prognostic and therapeutic target in HCC. In addition, the exposure of environmental pollutants will also trigger and exacerbate the occurrence of liver cancer by inducing the ISX and relevant epigenetic genes expression via the function of its upstream regulatory gene-AHR. At the same time, the expression of ISX in HCC cells can also inhibit the host's own immune system through the regulation of immune checkpoint and tryptophan metabolism-related genes to promote the further growth and metastasis of hepatoma cells. Here, we show that P300/CBP-associated factor (PCAF)-dependent acetylation of intestine-specific homeobox (ISX) regulates epithelial-mesenchymal transition (EMT) and promotes cancer metastasis. Mechanistically, PCAF acetylation of ISX at lysine 69 promotes the interaction with acetylated bromodomain-containing protein 4 (BRD4) at lysine 332 in tumor cells, and the translocation of the resulting complex into the nucleus. There, it binds to promoters of EMT genes, where acetylation of histone 3 at lysines 9, 14, and 18 initiates chromatin remodeling and subsequent transcriptional activation. Ectopic ISX expression enhances EMT marker expression, including TWIST1, Snail1, and VEGF, induces cancer metastasis, but suppresses E-cadherin expression. In lung cancer, ectopic expression of PCAF–ISX–BRD4 axis components correlates with clinical metastatic features and poor prognosis. These results suggest that the PCAF–ISX–BRD4 axis mediates EMT signaling and regulates tumor initiation and metastasis.
Full paper linkage: https://pubmed.ncbi.nlm.nih.gov/31908141/
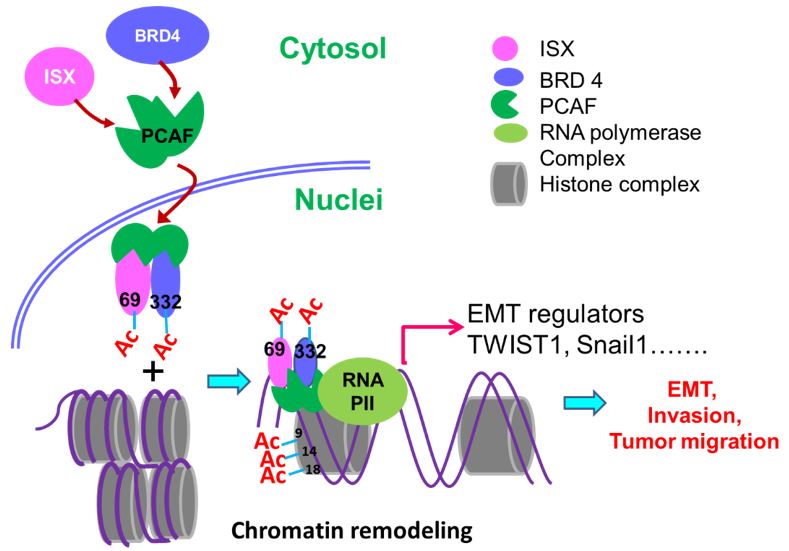
Figure legend: Illustration of experimental data and structure modeling, it is demonstrated that Tyr390 and Asn433 residues on the BD2 domain of BRD4 are critical residues needed for BRD4 to recognize and bind with the acetylated Lys69 of ISX to facilitate the formation of the ISX–BRD4 complex.
Main researcher Intro.
Professor Hsu Shih-Hsien, Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Author Email
Professor Hsu: jackhsu@kmu.edu.tw, TEL:07-3121101#2136
Paper cited from:
EMBO Rep. 2020 Feb 5;21(2):e48795.
Research Paper available online on website:
https://pubmed.ncbi.nlm.nih.gov/31908141/


