顎骨藥物性骨壞死
顎骨藥物性骨壞死
此案例為54歲乳癌女性合併多處骨轉移,在接受骨針Prolia TM(Denosumab) 治療,120mg每四周注射一次。經過10個月的治療後,產生嚴重的副作用-藥物性骨壞死(Medication related osteonecrosis of the Jaw): 造成顎骨持續疼痛,牙齒掉落,顎骨壞死暴露,嚴重感染的情形。爾後經過長期抗生素使用,腐骨清除術及自體頰脂肪墊移植後才改善其症狀。
骨質疏鬆症患者,Paget’s disease,多發性骨髓癌,乳癌,攝護腺癌及肺癌患者產生遠端骨轉移後,會長期接受使用雙磷酸鹽類或denosumab治療預防骨折產生。雙磷酸鹽為抗流失類藥物(antiresorptive agent):抑制蝕骨細胞活性,降低骨代謝率,減少骨質的溶蝕。Denosumab是一種人類RANKL的單株抗體,RANKL是由造骨細胞分泌的活化因子,會與蝕骨細胞結合使其成熟,而人造的單株抗體denosumab可與RANKL結合,干擾蝕骨細胞成熟,進而達到抑制骨質流失的目的。
研究顯示施打骨針後產生MRONJ的機率大約5/100,目前顎骨壞死並沒有標準有效的治療方法,主要以抗菌漱口水、藥物治療做症狀控制降低感染和減緩疼痛為主,手術清創去除壞死組織及骨骼為第二線的處置方式。然而預防勝於治療,在病人施打骨針前,應當先至口腔顎面外科或是牙科,做好口腔衛教及照護,拔除未來預後不良的牙齒。如果在藥物治療期間需要進行侵入性牙科手術,應請口腔外科醫師與病人溝通做審慎評估及處置。
醫師與病人都應當了解骨針藥物會帶來的副作用,共同合作來改善病人生活品質。
圖形摘要
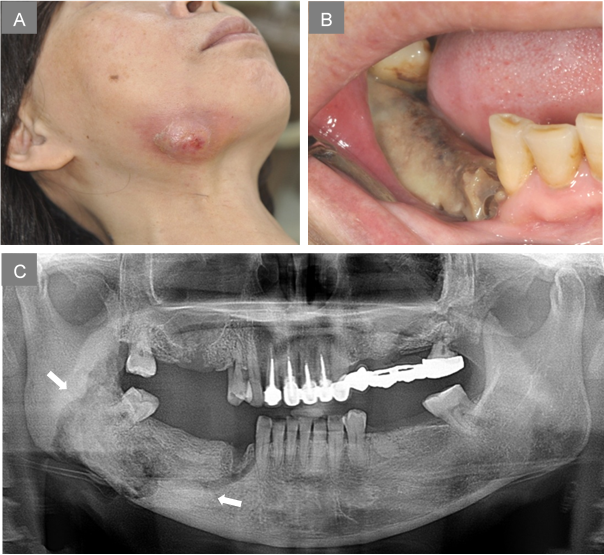
圖A 右下顎嚴重感染腫脹及膿瘍/圖B右下顎骨暴露,腐骨壞死/圖C.X光片顯示右下顎骨大範圍壞死,牙齒掉落
應用與亮點:
1. 使用骨針藥物治療癌症及骨質疏鬆症患者,需注意藥物性骨壞死的重要性。
2. 目前仍沒有標準的治療方式,期許未來有更多的研究投入。
【研究團隊】
團隊成員:陳裕豐、張宏博
代表單位:高雄醫學大學口腔醫學院
研究聯繫Email:omsyfchen@gmail.com
【論文資訊】
論文出處:期刊名. 年份月份; 期(卷): 頁數.
New England Journal of Medicine.2023 May 25;388:e69
全文下載:
https://www.nejm.org/doi/10.1056/NEJMicm2209172
Medication-Related Osteonecrosis of the Jaw
Medication-Related Osteonecrosis of the Jaw
A 54-year-old woman with breast cancer and multiple bone metastases who underwent treatment with ProliaTM (Denosumab) injection, administered at a dose of 120mg every four weeks. Severe side effects (1. necrotic jaw bone exposure, 2. spontaneous teeth loss, 3. severe pain, 4. persisted infection and extraoral fistula) happened after 10 months of treatment. Medication-related osteonecrosis of the Jaw (MRONJ) was diagnosed. We successfully treated this patient with long-term antibiotics, surgical debridement with sequestrectomy, autologous buccal fat pad transplantation.
Patients with osteoporosis, Paget’s disease, multiple myeloma, breast cancer, prostate cancer, and lung cancer with distant bone metastases often receive long-term bisphosphonates or denosumab treatment to prevent fractures. Bisphosphonates are antiresorptive agents: they inhibit osteoclast activity, reduce bone turnover, and decrease bone resorption. Denosumab is a monoclonal antibody against human RANKL, an activator secreted by osteoblasts that binds to osteoclasts, promoting their maturation. Denosumab interferes with this process by binding to RANKL, thereby inhibiting osteoclast maturation and preventing bone loss.
Studies presented that the incidence of MRONJ is approximately 5/100. There is no standard effective treatment protocol for MRONJ. Conservative treatment using antimicrobial mouthwashes and antibiotics is the first line treatment for symptoms control to reduce infection and pain. Surgical debridement to remove necrotic tissue and bone is the second line treatment option.
However, prevention is better than cure. Before initiating bone agent therapy, patients should consult with oral and maxillofacial surgeons or dentists for oral health education and care. Poor prognoses teeth should be extracted preemptively. If invasive dental procedures are necessary during drug treatment, careful assessment and management should be carried out in collaboration with oral and maxillofacial surgeons.
Both physicians and patients should understand the side effects associated with bone agents and work together to improve patients' quality of life.
Graphical Abstract:

Image A: Severe infection with extraoral skin fistula over right mandibular angle./Image B: Necrotic bone exposure and premolar loss over right mandible./ Image C: Extensive jaw bone necrosis over right mandible.
Application and Highlights:
1.It is important to be mindful of the significance of medication-related osteonecrosis when treating cancer and
osteoporosis patients with bone agents.
2.Currently, there is still no standard treatment method, and it is hoped that there will be more research efforts in the future.
Research Team Members: Yu-Feng Chen, Hong-Po Chang
Representative Department: College of Dental Medicine, Kaohsiung Medical University
Contact Email: omsyfchen@gmail.com
Publication: New England Journal of Medicine.2023 May 25;388:e69
Full-Text Article: https://www.nejm.org/doi/10.1056/NEJMicm2209172

