細胞自噬維持「微小RNA」的穩態平衡可防止癌症之發生
細胞自噬維持「微小RNA」的穩態平衡可防止癌症之發生
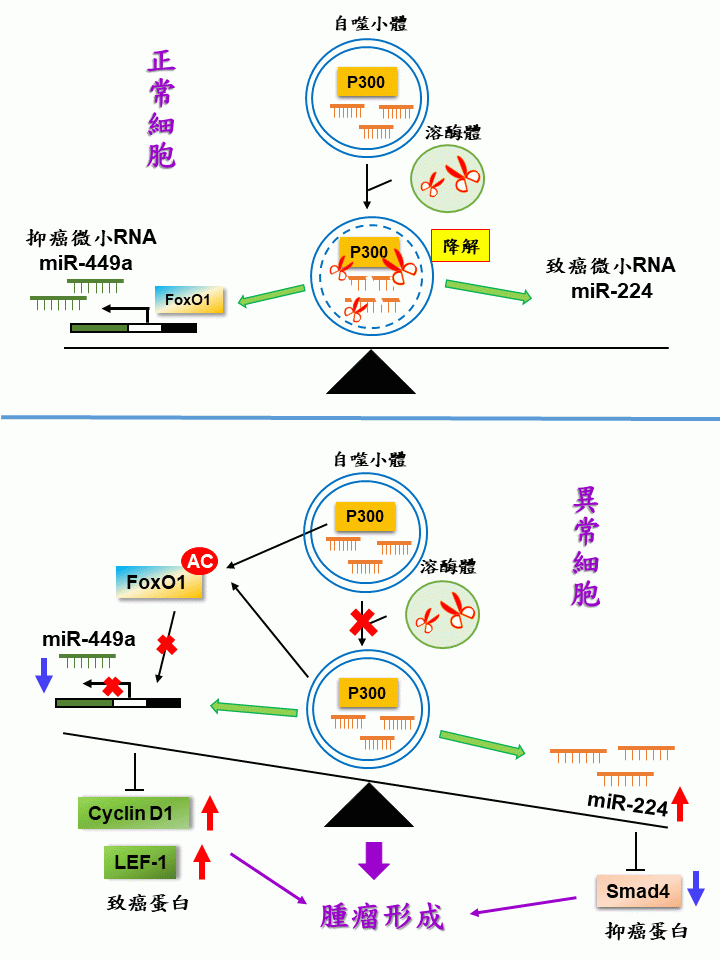
細胞自噬在維持胞內各種功能之平𧗾與穩定至關重要,適當的自噬反應經由降解機制保護細胞免受各種壓力造成的損傷。自噬功能障礙導致各種疾病,包括神經退化性疾病與癌症。微小RNA(miRNA)由20~23個非編碼RNA核苷酸所組成。主要是降解相對應的訊息RNA或阻止其轉譯來調節胞內基因之表達。由於自噬與微小RNA均與腫瘤之發生有關,兩者間的關係值得探討。許多研究探討微小RNA如何調節自噬來影響癌症的發展,很少研究探討自噬如何調節微小RNA影響腫瘤之發生。我們率先報導自噬可以負向調控微小RNA miR-224以及正向調控微小RNA miR-449a來影響腫瘤的發展。自噬選擇性的將miR-224送到自噬小體(autophagosome)進行降解,來負向調控miR-224並導致目標基因Smad4之過量表達,從而抑制肝癌腫瘤細胞之爬行與增生 (Hepatology, 2014)。我們最近的研究揭露自噬正向調控miR-449a之成果發表在學術期刊(Frontiers in Oncology, 2021)。在大腸直腸癌中,細胞自噬選擇性地降解「組蛋白乙醯轉移酶」p300,該蛋白負責轉錄FOXO1蛋白的乙醯化,並阻止其從細胞質遷移到細胞核去驅動miR-449a之表達。在大腸直腸癌的癌化過程中,由於自噬相關基因受到抑制,導致miR-449a的表達被抑制以及兩個目標基因LEF-1和cyclinD1的過度表達,進而誘發大腸直腸癌的發生。雖然細胞自噬降解系統可同時調節細胞中miR-224和miR-449a的平衡,但其作用機制不同。前者直接影響miR-224之表現量,但後者影響miR-449a上游基因之轉錄。我們已証實在肝癌和大腸直腸癌中均有低自噬活性、高 miR-224 以及低 miR-449a表達的現象。綜上所述,我們在肝癌和大腸直腸癌中揭露了自噬活性同時與兩種微小RNA之間的關聯。自噬同時平衡miR-224的降解與miR-449a的合成,以適應外界的壓力並維持胞內微小RNA的平衡與穩態。上述任何一個或兩個微小RNA調節失去平衡即會觸發腫瘤之發生。我們的創新發現證實了自噬降解系統經由正向與負向調控微小RNA之平衡以維持細胞正常功能來避免癌症發生的可能性,同時開啟了調節細胞自噬活性可控制癌症進展的新思維。
圖形摘要
【應用與亮點】
1. 揭露大腸直腸癌的癌化過程中,由於自噬受到抑制,導致miR-449a的表達被抑制以及兩個目標基因LEF-1和cyclinD1的過度表達,進而誘發大腸直腸癌的發生
2. 揭露自噬同時平衡miR-224的降解與miR-449a的合成以避免肝癌和大腸直腸癌之發生。
【研究團隊】
團隊成員:劉校生、林姵妏、朱曼菱、劉玉雯、藍昇輝、吳珊瑩
代表單位:高雄醫學大學癌症研究中心及醫學院熱帶醫學碩士學位學程
團隊簡介:本團隊於癌症研究中心負責細胞自噬分析平台之建立,研究各種癌症及病毒感染與細胞自噬的相互關係及可能的作用機制,最終目標為尋求調節細胞自噬活性維持細胞生理機能之平衡減緩疾病病情之新頴策略。
◆劉校生:高雄醫學大學癌症研究中心之成員及高雄醫學大學熱帶醫學碩士學程客座特聘教授
◆林姵妏:高雄醫學大學癌症研究中心之成員及高雄醫學大學熱帶醫學碩士學程博士後研究員
◆朱曼菱:高雄醫學大學熱帶醫學碩士學程研究助理
◆劉玉雯:高雄醫學大學癌症研究中心之成員及高雄醫學大學熱帶醫學碩士學程研究助理
◆藍昇輝:國立陽明交通大學生生命科學系及基因體中心助理教授
◆吳珊瑩:台北醫學大學微生物及免疫學系助理教授
研究聯繫Email:hsliu@kmu.edu.tw
【論文資訊】
論文出處:Frontiers in Oncology, 2021, Oct.; 11: 738144
全文下載:https://doi.org/10.3389/fonc.2021.738144
Autophagy maintains homeostasis and balance of microRNAs to prevent cancer occurrence
Autophagy maintains homeostasis and balance of microRNAs to prevent cancer occurrence
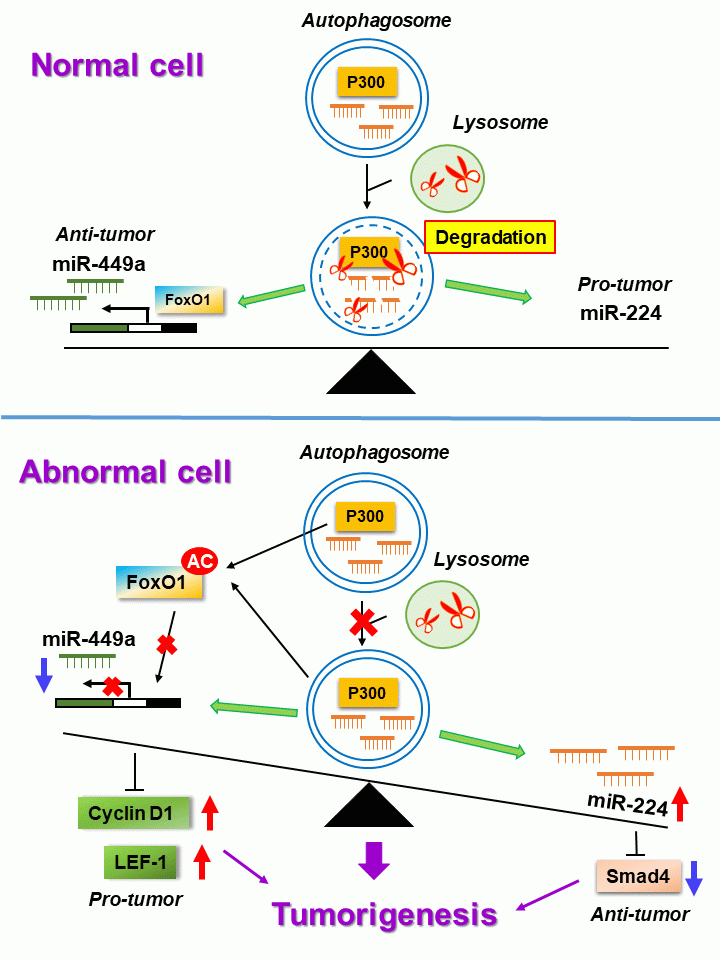
Autophagy plays an essential role in maintaining cellular homeostasis to protect cells from stress-caused damage through the degradation machinery. Dysfunction of autophagy contributes to various human diseases including neurodegenerative disorders and cancers. MicroRNAs (miRNAs) are 20~23 nucleotide non-coding RNAs. Most of them regulate gene expression either by degrading target RNAs or by blocking translation through complementary binding with the 3′ untranslated region (3′ UTR) of the target RNA. MiRNAs participate in diverse biological functions for cell differentiation, proliferation, longevity, and diseases including cancers. Increasing evidence indicate that autophagy plays an anti-tumor role in the early stage of tumorigenesis. Conversely, it shifts to pro-tumor role in late stages of tumorigenesis. Similarly, miRNAs act either as a tumor suppressor or as an oncogene based on the genes they target in different cancers. Since both autophagy and miRNAs participate in tumorigenesis, their relationship warrants extensive exploration. Notably, many studies investigated how miRNAs regulate autophagy-related genes to affect cancer development. In contrast, very few studies explore how autophagy regulates miRNAs to affect tumorigenesis. Therefore, we developed a cell-line system to screen autophagy regulated miRNAs which are involved in tumor formation. We identified one autophagy negatively regulated miRNA (miRNA-224) and one positively regulated miRNA (miR-449a) using miRNA microarray. For the miRNA negatively regulated by autophagy, miR-224 is preferentially delivered to the autophagosome for degradation, which results in upregulation of Smad4 to suppress liver cancer formation (HCC) (Hepatology, 2014).
For the miRNA positively regulated by autophagy, we revealed that in colorectal cancer (CRC) tumors, autophagy selectively degrades the coactivator p300 histone acetyltransferase, which is responsible for acetylation of Forkhead box protein O1 (FOXO1), as well as prevents its migration from the cytoplasm to the nucleus. We confirmed that FoxO1, as a transcription factor, is responsible for the expression of miR-449a. Activation of autophagy promotes nuclear translocation of FoxO1 followed by binding with the miR-449a promoter to trigger miR-449a transcription. Autophagy positively regulates miRNA expression through degradation of p300, which results in nuclear translocation of the un-acetylated FoxO1 to transcribe miR-449a gene. During the development of CRC, autophagic activity was decreased. This phenomenon results in the increase of p300 level and FoxO1 acetylation. Finally, the transcriptional level of miR-449a is suppressed, which results in overexpression of LEF-1 and cyclinD1 (target genes of miR-449a) followed by cancer development (Frontiers in Oncology, 2022). Low autophagic activity, high miR-224 as well as low expression of miR-449a in HCC and CRC have been reported. Accordingly, we confirmed the association between autophagic activity and two differentially expressed miRNAs in HCC and CRC cell lines. These findings imply that autophagy machinery simultaneously balances the degradation and synthesis of miR-224 and miR-449a, respectively, to maintain cellular homeostasis during adaptation to tumorigenic stresses. Dysfunction of regulation of either one or both miRNAs trigger tumors development. In conclusion, our findings open a new avenue toward manipulating autophagy degradation system to control the balance of miRNAs and maintain the normal functions of the cell.
Graphical Abstract
Application and Highlights:
1. Revealing that in CRC development, low autophagic activity results in low expression of miR-449a accompanied by overexpression of two target genes LEF-1 and cyclinD1 which trigger tumor formation and increased mobility.
2. Demonstrating how autophagy balances the degradation of miR-224 and the synthesis of miR-449a to avoid the occurrence of liver and colorectal cancer.
Research Team Members: Hsiao-Sheng Liu, Pei-Wn Lin, Man-Lin Chu, Yu-Wen, Liu, Sheng-Hui Lan, and Shan-Ying Wu
Representative Department:
◆Dr. Hsiao-Sheng Liu:
1. Center for Cancer Research, Graduate Institute of Clinical Medicine;
2. Master of Science Program in Tropical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung,
Taiwan
◆Dr. Pei-Wen Lin:
1. Center for Cancer Research, Graduate Institute of Clinical Medicine;
2. Master of Science Program in Tropical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung,
Taiwan
◆Man-Lin Chu:
Master of Science Program in Tropical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung,
Taiwan
◆Yu-Wen Liu:
1. Center for Cancer Research, Graduate Institute of Clinical Medicine;
2. Master of Science Program in Tropical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung,
Taiwan
◆Dr. Sheng-Hui Lan:
Department of Life Science and Institute of Genome Sciences, National Yang Ming Chiao Tung University, Taipei,
Taiwan
◆Dr. Shan-Ying Wu:
Department of Microbiology and Immunology, College of Medicine, Taipei Medical University, Taipei, Taiwan
Introduction of Research Team:
The mission of this team is to establish an autophagy analysis platform at the Center of Cancer Research, KMU to study the interrelationship and possible mechanisms of action between various cancers and viral infections and autophagy. The ultimate goal is to develop novel strategies to maintain the homeostasis of cell physiological functions and the balance of miRNAs to alleviate disease symptoms through manipulating autophagy activity.
Contact Email: hsliu@kmu.edu.tw


