世界首創C型肝炎簡易治療演算法,適合所有領域醫師加入治療C型肝炎行列-台灣研究可望擴大C型肝炎病患之治療照護加速WHO消除C型肝炎之目標
世界首創C型肝炎簡易治療演算法,適合所有領域醫師加入治療C型肝炎行列-台灣研究可望擴大C型肝炎病患之治療照護加速WHO消除C型肝炎之目標
抗病毒C型肝炎全口服新藥的問世為C型肝炎治療帶來革命性的突破,但仍有一些病人因缺乏專科醫療資源而無法接受治療。為順利達成世界衛生組織訂定2030年消除C型肝炎目標,各國莫不致力於將專科醫師以外其他科別醫師納入C型肝炎照護的行列,積極推動去中心化和分工治療的政策。
國內一項由高雄醫學大學余明隆講座教授和國內53家醫療院所合作的研究,針對台灣肝臟研究學會C型肝炎登錄計畫(TACR)收錄的7,677位接受泛基因型全口服新藥sofosbuvir/velpatasvir或glecaprevir/pibrentasvir的C型肝炎病患進行觀察分析,發現符合歐美肝病醫學會建議非專科醫師治療條件的病人,其肝指數異常升高比率雖然非常低,但經多變項分析發現,年齡大於70歲、肝癌病史、總膽紅素大於1.2 mg/dL、腎絲球過濾率小於60 mL/min/1.73m2 和FIB-4大於3.25仍是肝指數異常升高(總膽紅素 total bilirubin, AST, ALT)的危險因子。根據以上結果,該團隊提出TACR演算法,提供明確的非專科醫師照護的病人條件。研究結果已刊登在最新一期的國際期刊Hepatology International。
研究團隊表示,這項創新的TACR演算法,根據臨床特徵識別出適合簡化治療的C型肝炎病人,只要符合條件就可由非專科醫師開立全口服新藥進行治療,對於某些有較高風險但可以控制的病人建議轉介至專科醫師評估後,可以再回到非專科醫師開立全口服新藥進行治療追蹤照護;至於那些有更高風險的病人則建議轉介至專科醫師追蹤照護;未來這套演算法將可望推動C型肝炎治療的普及,並大幅提高治療涵蓋率,為台灣邁向2025年消除C型肝炎的目標再邁進一大步。
圖形摘要
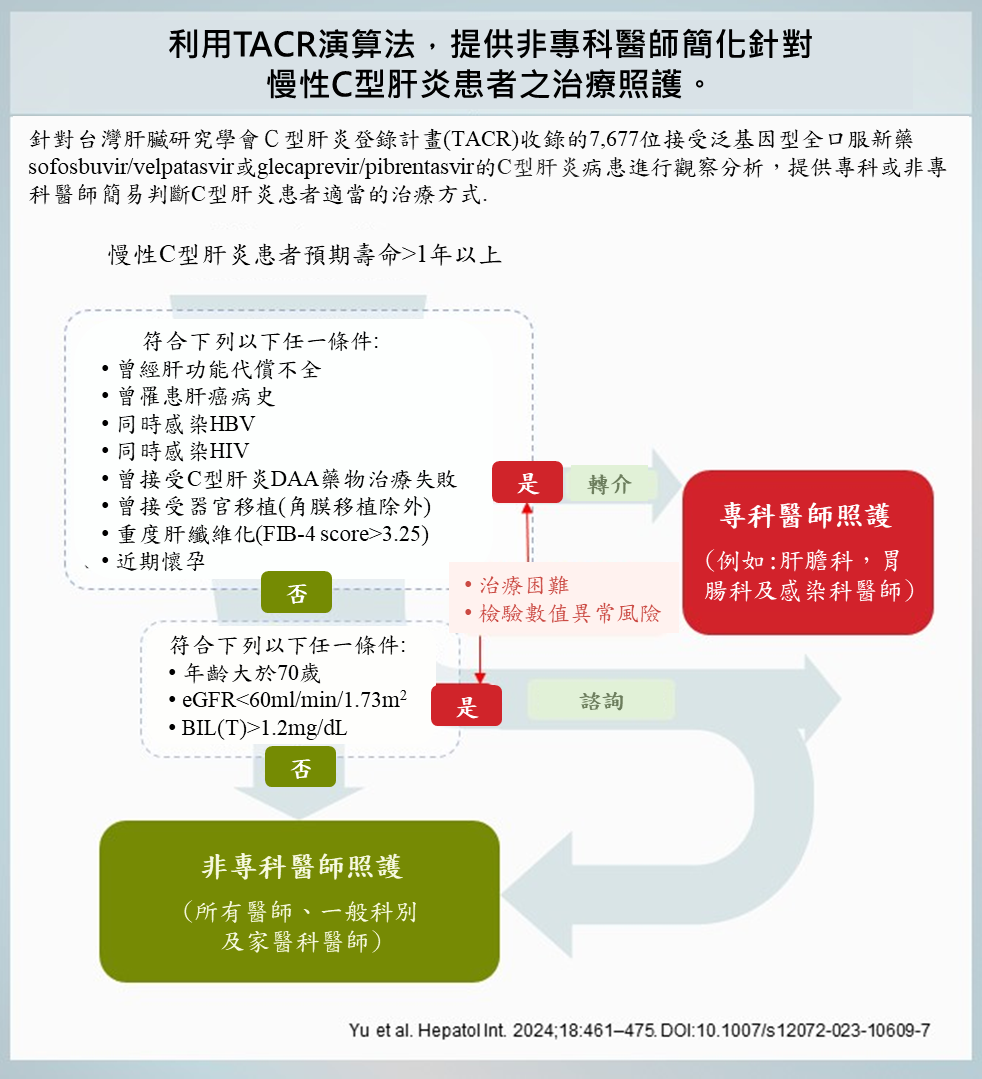
應用與亮點:
1.開發創新的 TACR 演算法,可根據臨床特徵識別適合簡化治療的C型肝炎患者。
2.根據TACR 演算法符合條件的C型肝炎患者,就可以接受由非專科醫生開具的泛基因型全口服新藥
sofosbuvir/velpatasvir或glecaprevir/pibrentasvir進行治療。
3. 這套演算法將可望推動C型肝炎治療的普及,並大幅提高治療涵蓋率,為台灣邁向2025年消除C型肝炎的
目標再邁進一大步。
【研究團隊】
團隊成員:余明隆講座教授、莊萬龍教授、戴嘉言教授、黃志富教授、黃釧峰教授、葉明倫教授。
代表單位:液態生物檢體暨世代研究中心及醫學院肝炎研究中心
團隊簡介:余明隆講座教授及莊萬龍教授研究團隊,皆為高雄醫學大學附設中和紀念醫院肝膽胰內科主治醫師,同時也是醫學院肝炎研究中心及液態生物檢體暨世代研究中心的成員,致力於肝臟疾病之病因、致病機轉、治療及預防的研究及服務。
研究聯繫Email: fish6069@gmail.com
【補充資訊】
患者納入分析期間為2019年8月到2021年8月
【論文資訊】
論文出處: Yu ML, et al. An algorithm for simplified hepatitis C virus treatment with non-specialist care based on nation-wide data from Taiwan. Hepatol Int. 2024 Apr;18(2):461-475.
全文下載: https://link.springer.com/article/10.1007/s12072-023-10609-7
An algorithm for simplified hepatitis C virus treatment with non-specialist care based on nation-wide data from Taiwan
An algorithm for simplified hepatitis C virus treatment with non-specialist care based on nation-wide data from Taiwan
Background: Both European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD-IDSA) guidelines recommend simplified hepatitis C virus (HCV) treatment with pan-genotypic sofosbuvir/velpatasvir or glecaprevir/pibrentasvir for eligible patients. This observational study used real-world data to assess these regimens' safety in eligible patients and develop an algorithm to identify patients suitable for simplified treatment by non-specialists.
Methods: 7,677 HCV-infected patients from Taiwan Registry (TACR) who received at least one dose of sofosbuvir/velpatasvir or glecaprevir/pibrentasvir and fulfil led the EASL/AASLD-IDSA criteria for simplified treatment were analyzed. Multivariate analysis was conducted on patient characteristics and safety data.
Results: Overall, 92.8% (7,128/7,677) of patients achieved sustained virological response and only 1.9% (146/7,677) experienced Grades 2-4 laboratory abnormalities in key liver function parameters (alanine aminotransferase, aspartate aminotransferase, and total bilirubin), with only 18 patients (0.23%) experiencing Grades 3-4 abnormalities. Age > 70 years old, presence of hepatocellular carcinoma, total bilirubin > 1.2 mg/dL, estimated glomerular filtration rate < 60 mL/min/1.73 m2, and Fibrosis-4 > 3.25 were associated with higher risks of Grades 2-4 abnormalities. Patients with any of these had an odd of 4.53 times than that of those without in developing Grades 2-4 abnormalities (p < 0.01).
Conclusions: Real-world data from Taiwan confirmed that simplified HCV treatment for eligible patients with pan-genotypic regimens is effective and well tolerated. The TACR algorithm, developed based on this study's results, can further identify patients who can be safely managed by non-specialist care.
Graphical Abstract
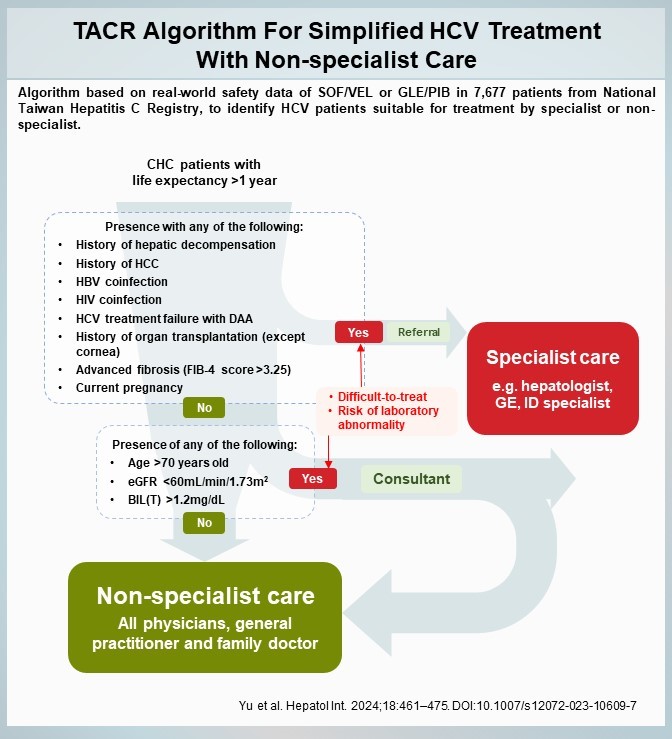
Implications and Application and Highlights:
1.This is an innovative TACR algorithm that identifies patients with hepatitis C who are suitable for simplified treatment based on their clinical characteristics.
2.Candidates with hepatitis C may receive pan-genotypic sofosbuvir/velpatasvir or glecaprevir/pibrentasvir prescribed by a non-specialist physician as long as they are eligible.
3.In the future, this algorithm is expected to promote the popularization of hepatitis C treatment and significantly increase treatment coverage, taking a big step towards the goal of eliminating hepatitis C in Taiwan by 2025.
Research Team Members:
Ming-Lung Yu, Wan-Long Chuang, Chia-Yen Dai, Jee-Fu Huang, Chung-Feng Huang, Ming-Lun Yeh.
Representative Department:
Center for Liquid Biopsy and Cohort Research, Kaohsiung Medical University, and Hepatitis Research Center, College of Medicine, Kaohsiung Medical University.
Introduction of Research Team:
Professor Ming-Lung Yu and Wan-Long Chuang are members of the Hepatitis Center and Hepatobiliary Division, Department of Internal Medicine, Kaohsiung Medical University Hospital and Hepatitis Research Center, College of Medicine, Kaohsiung Medical University, and Center for Liquid Biopsy and Cohort Research, Kaohsiung Medical University. They dedicate to the research and service of Liver diseases, including etiologies, epidemiology, pathogenesis, therapeutic modalities, and prevention of hepatitis B, hepatitis C, steatohepatitis, and liver cancers.
Contact Email: fish6069@gmail.com
Publication: Hepatol Int.2024 Apr;18(2):461-475.
Full-Text Article: https://link.springer.com/article/10.1007/s12072-023-10609-7


